Recapping the Planck Formula Remember Light gamma rays

Recapping the Planck Formula

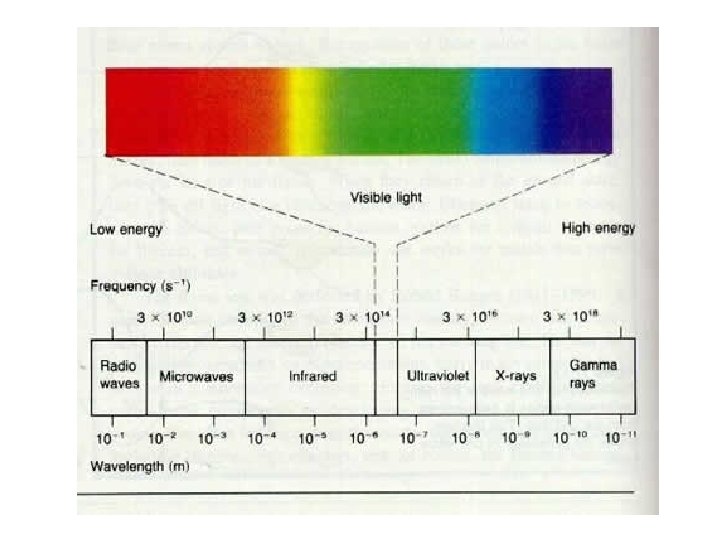
Remember Light, gamma rays, microwaves etc are all electromagnetic waves. • An electromagnetic wave is made up a stream of particles called photons. • Each photon has no mass but has a fixed amount of energy. • The energy of the photon depends on the frequency of the light
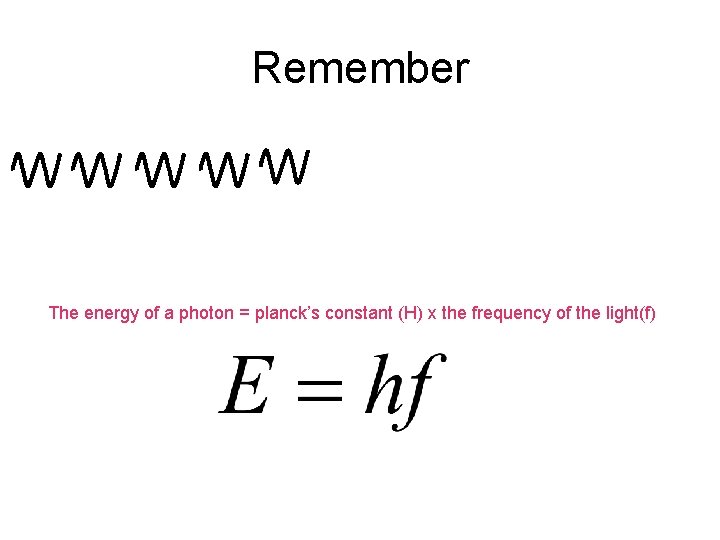
Remember The energy of a photon = planck’s constant (H) x the frequency of the light(f)
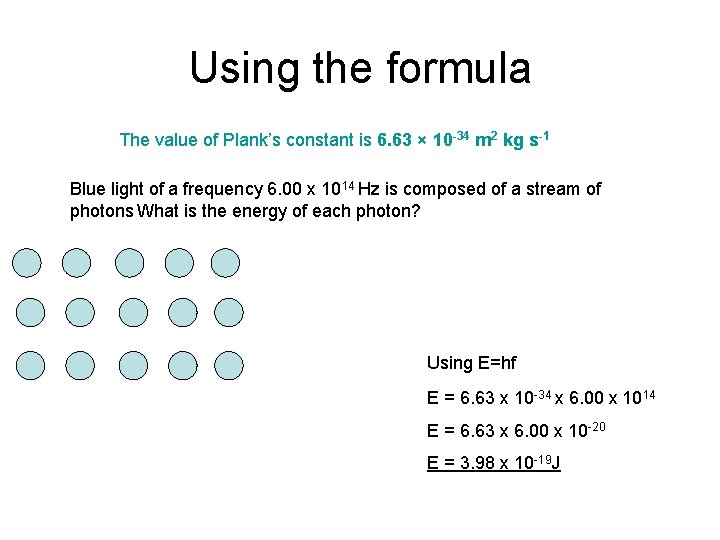
Using the formula The value of Plank’s constant is 6. 63 × 10 -34 m 2 kg s-1 Blue light of a frequency 6. 00 x 1014 Hz is composed of a stream of photons What is the energy of each photon? Using E=hf E = 6. 63 x 10 -34 x 6. 00 x 1014 E = 6. 63 x 6. 00 x 10 -20 E = 3. 98 x 10 -19 J
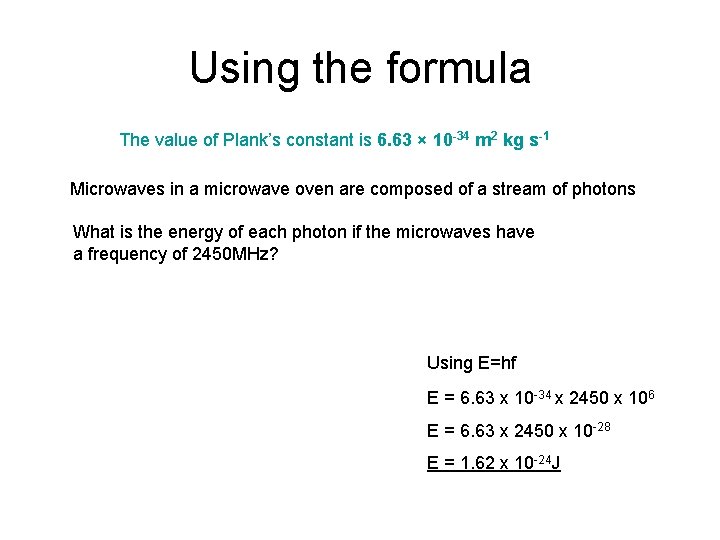
Using the formula The value of Plank’s constant is 6. 63 × 10 -34 m 2 kg s-1 Microwaves in a microwave oven are composed of a stream of photons What is the energy of each photon if the microwaves have a frequency of 2450 MHz? Using E=hf E = 6. 63 x 10 -34 x 2450 x 106 E = 6. 63 x 2450 x 10 -28 E = 1. 62 x 10 -24 J
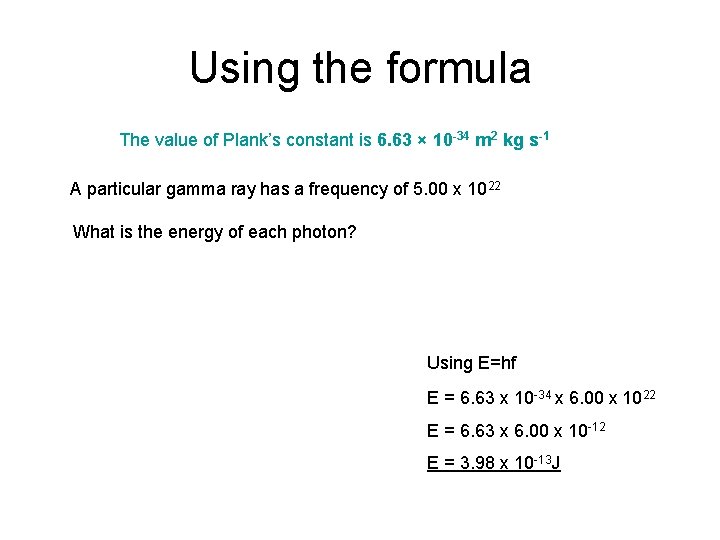
Using the formula The value of Plank’s constant is 6. 63 × 10 -34 m 2 kg s-1 A particular gamma ray has a frequency of 5. 00 x 1022 What is the energy of each photon? Using E=hf E = 6. 63 x 10 -34 x 6. 00 x 1022 E = 6. 63 x 6. 00 x 10 -12 E = 3. 98 x 10 -13 J
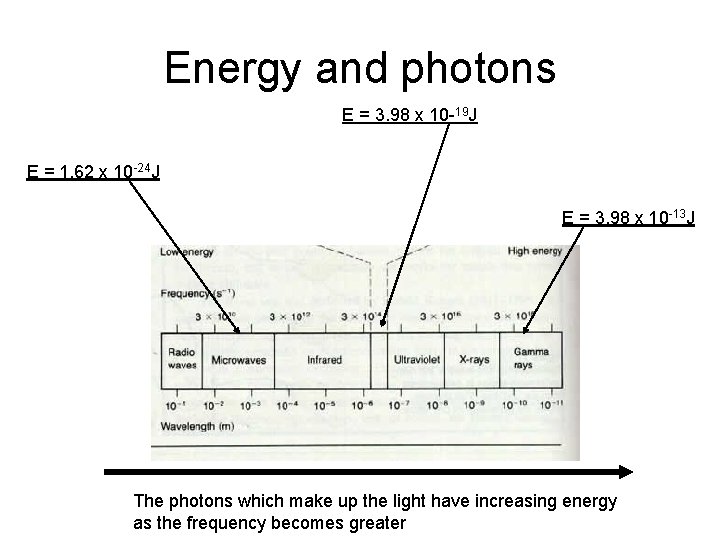
Energy and photons E = 3. 98 x 10 -19 J E = 1. 62 x 10 -24 J E = 3. 98 x 10 -13 J The photons which make up the light have increasing energy as the frequency becomes greater
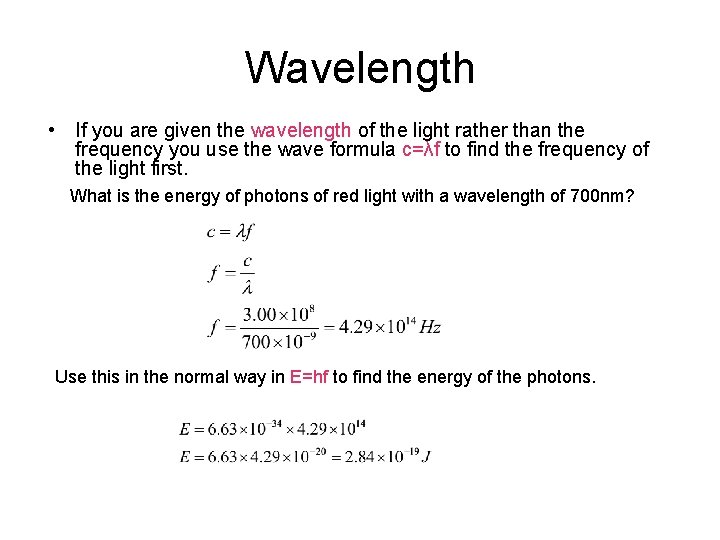
Wavelength • If you are given the wavelength of the light rather than the frequency you use the wave formula c=λf to find the frequency of the light first. What is the energy of photons of red light with a wavelength of 700 nm? Use this in the normal way in E=hf to find the energy of the photons.
- Slides: 9