REAUDIT Radical ChemoRadiotherapy for Oesophageal Cancer An audit














- Slides: 16

RE-AUDIT Radical Chemo-Radiotherapy for Oesophageal Cancer: An audit of dose-fractionation schedules and timeliness of treatment Dr L Dixon and Dr J Wadsley Department of Clinical Oncology, Weston Park Hospital, Sheffield, UK.

Background Chemo-radiotherapy is a radical treatment option for patients with oesophageal cancer. RCR guidelines 1, include both squamous cell carcinoma and adenocarcinoma of the oesophagus as Category 1 tumour types. • RCR Dose Fractionation Guidelines 2006 � 50. 4 Gy in 28 fractions, or 50 Gy in 25 fractions. • Current local guidelines � 50 Gy in 25 fractions.

Aims • Primary aim - to audit the timeliness and dose fractionation schedule of radical chemo-radiotherapy for oesophageal cancer • Secondary aim - to assess the survival following treatment: � median survival � 1 -year and 2 -year overall survival • Initial Audit: 1 st Sept 2004 – 31 st Jan 2013 • Re-Audit: 1 st Feb 2013 – 31 st Jan 2015

Standards 1. Most patients should have dose fractionation schedules which comply with RCR guidelines 2 2. At least 95% of patients should not have a prolongation of overall treatment time in excess of two days 1 3. There is no possible standard for mortality figures.

Methods • Retrospective audit • Electronic search identified patients treated • Initial Audit: 52 patients included • Re-audit: 17 patients included • Analysis was performed using MS Excel and Kaplan. Meier survival was generated Graph. Pad Prism.

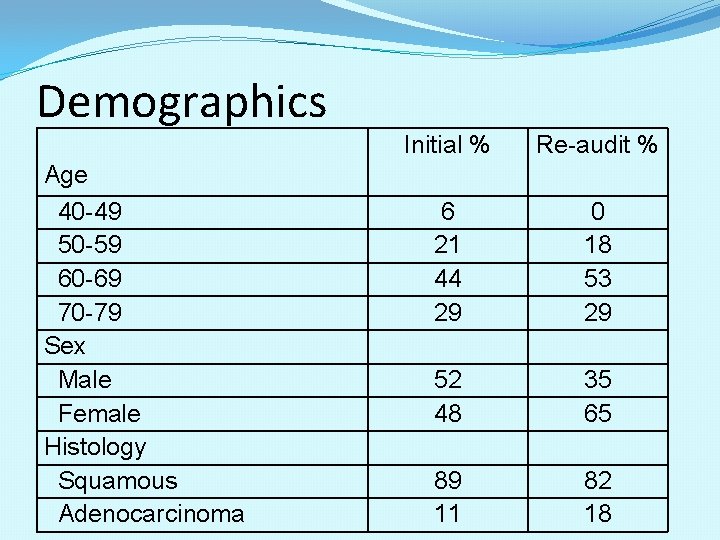
Demographics Age 40 -49 50 -59 60 -69 70 -79 Sex Male Female Histology Squamous Adenocarcinoma Initial % Re-audit % 6 21 44 29 0 18 53 29 52 48 35 65 89 11 82 18

Results – Initial Audit 1. Achieved: 94% (49) received a dose fractionation schedules in accordance with RCR guidelines 2. � These patients received 50 Gy in 25# 2. Not achieved: 85% (44) completed treatment without excessive prolongation of overall treatment time 3. Median Survival 26. 1 months Overall 2 -year survival 56%

Discussion – Initial Audit Overall Treatment Time � 8 (15%) patients had excessive prolongations � 7 patients - not treated on bank holidays � 1 patient - 5 day gap due to oesophageal stent insertion; gap calculation was performed to compensate (54 Gy in 27#) � RCR guidance 1 published December 2008 � Post Dec 2008 - 94% (30/32) had no prolongation in excess of two days � 2 patients - not treated bank holidays � Both patients identified as Category 2 by clinician

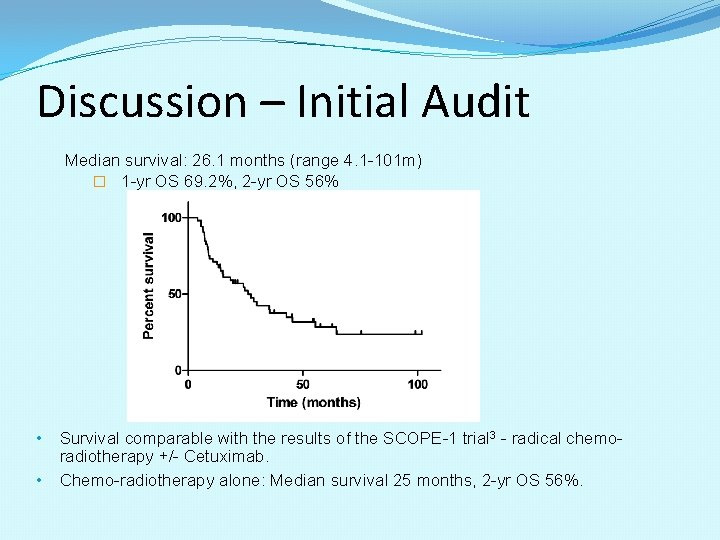
Discussion – Initial Audit Median survival: 26. 1 months (range 4. 1 -101 m) � 1 -yr OS 69. 2%, 2 -yr OS 56% • Survival comparable with the results of the SCOPE-1 trial 3 - radical chemoradiotherapy +/- Cetuximab. • Chemo-radiotherapy alone: Median survival 25 months, 2 -yr OS 56%.

Re-Audit • Initial results presented locally, to increase awareness regarding Category 1 status for all radically treatable oesophageal cancers. • Education of medical staff and radiotherapy department regarding avoiding delays to treatment for Category 1 patients. • Results presented nationally at RCR Clinical Oncology Audit Meeting 2014.

Results – Re-audit 1. Achieved: 100% (17) received a dose fractionation schedules in accordance with RCR guidelines 2. � These patients received 50 Gy in 25# 2. Achieved: 100% (17) completed treatment without excessive prolongation of overall treatment time

Discussion – Re-audit � Improvement seen since initial audit and presentation to local department � Re-education beneficial in helping to ensure patients identified correctly as category 1 treatment types. � Both targets now achieved in re-audit

Possible Future Plans • Project underway comparing outcomes between chemo-radiotherapy and radical radiotherapy alone • Further re-audit to review progress and ensure standards are being maintained. • Review survival – disease related or other causes • Review tolerability of treatment • Toxicity and long term effects

Conclusion �Chemo-radiotherapy remains a valid treatment option for oesophageal cancer �Squamous cell and adenocarcinomas should both be treated as category 1 tumour types, with no excessive prolongations to overall treatment time �Dose-fractionation schedules should be compliant with RCR Dose-Fractionation Guidelines 1

References 1. The timely delivery of radical radiotherapy: standards and guidelines for the management of unscheduled treatment interruptions, Third edition, 01 December 2008. RCR Guidelines. 2. RCR Dose Fractionation Guidelines, June 2006. 3. Chemoradiotherapy with or without Cetuximab in patients with oesophageal cancer (SCOPE 1): a multicentre, phase 2/3 randomised trial. Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI, Cunningham D, Blazeby J, Roy R, Maughan T, Griffiths G. Lancet Oncol. 2013 Jun; 14(7): 627 -37. doi: 10. 1016/S 1470 -2045(13)70136 -0. Epub 2013 Apr 25.

Thank you