Realtime Diagnostics of Jet Engine Exhaust Plumes Using


![References [1] Geoffrey Duxbury, “Infrared Vibration-Rotation Spectroscopy: From Free Radicals To The Infrared Sky”, References [1] Geoffrey Duxbury, “Infrared Vibration-Rotation Spectroscopy: From Free Radicals To The Infrared Sky”,](https://slidetodoc.com/presentation_image/047496446bfb2653486b9e14961ee13d/image-15.jpg)
- Slides: 15

Real-time Diagnostics of Jet Engine Exhaust Plumes, Using a Chirped QC Laser Spectrometer Kenneth G. Hay, Geoffrey Duxbury, and Nigel Langford Department of Physics, University of Strathclyde, John Anderson Building, 107 Rottenrow, Glasgow, G 4 0 NG, UK g. duxbury@strath. ac. uk Mark. P Johnson and John Black Rolls Royce Plc, PO Box 31, Derby DE 24 8 BJ June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

Outline of Talk • Intra-pulse Quantum Cascade laser spectrometer • Resolution of Intra-pulse spectrometers • Jet engine measurements • Future developments? June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

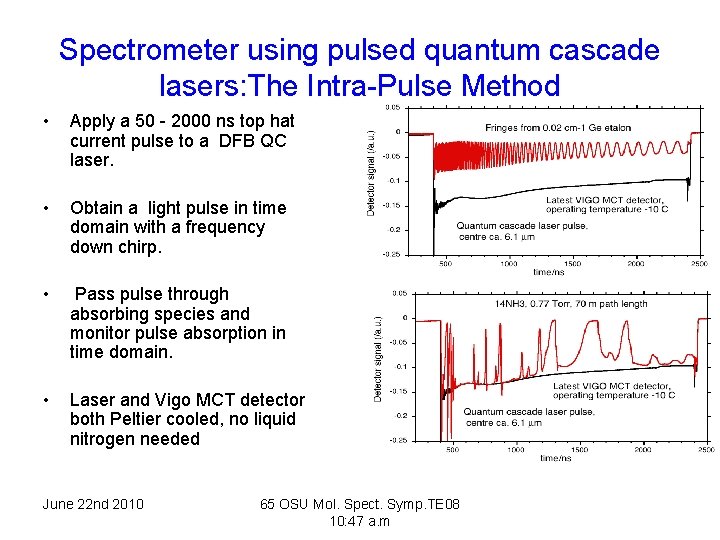
Spectrometer using pulsed quantum cascade lasers: The Intra-Pulse Method • Apply a 50 - 2000 ns top hat current pulse to a DFB QC laser. • Obtain a light pulse in time domain with a frequency down chirp. • Pass pulse through absorbing species and monitor pulse absorption in time domain. • Laser and Vigo MCT detector both Peltier cooled, no liquid nitrogen needed June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

Advantages of Intra-pulse Method • Real time display of absorption spectra. • Monitor time evolution of samples • Large frequency sampling window per pulse. • Multiple species detection and identification • • • Rapid collection of data Short pulse duration minimises vibration noise. Chirped pulse eliminates fringing in multiple pass cell. Top hat pulse generation straightforward. Simple. June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

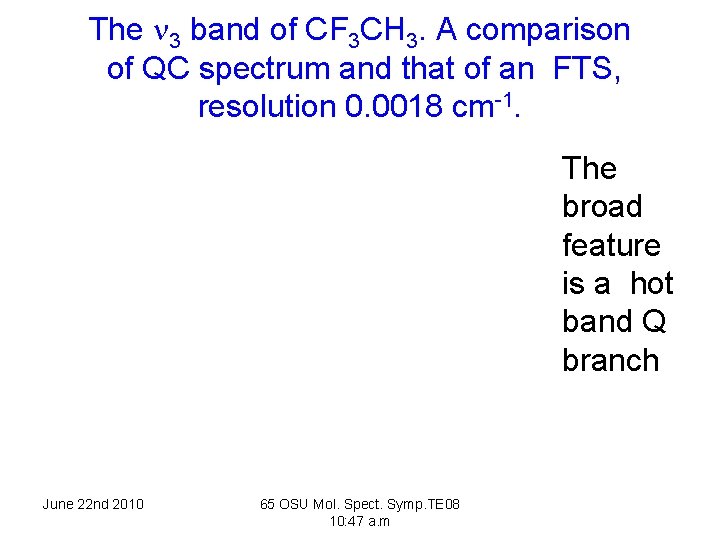
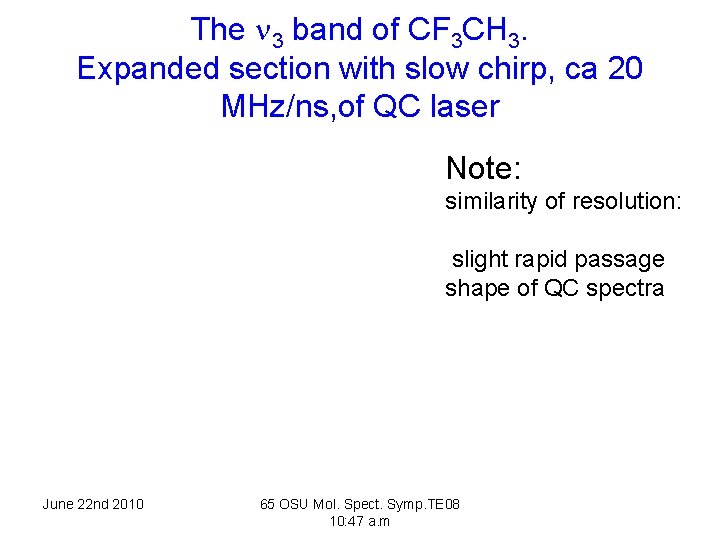
The 3 band of CF 3 CH 3. A comparison of QC spectrum and that of an FTS, resolution 0. 0018 cm-1. The broad feature is a hot band Q branch June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

The 3 band of CF 3 CH 3. Expanded section with slow chirp, ca 20 MHz/ns, of QC laser Note: similarity of resolution: slight rapid passage shape of QC spectra June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

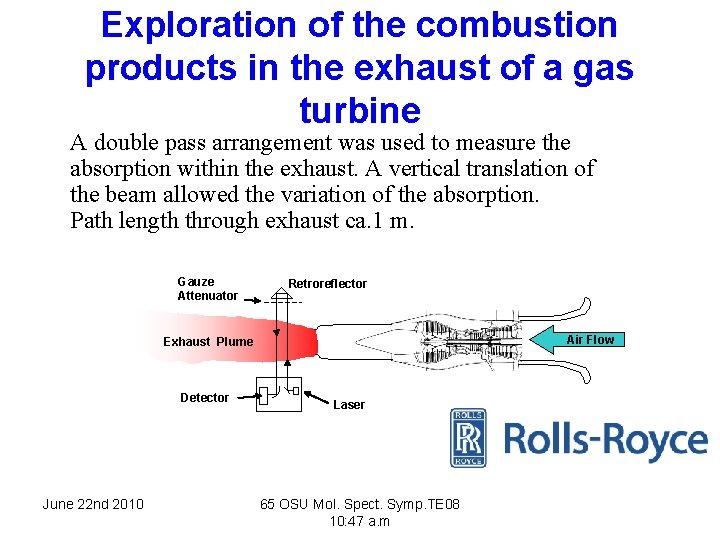
Exploration of the combustion products in the exhaust of a gas turbine A double pass arrangement was used to measure the absorption within the exhaust. A vertical translation of the beam allowed the variation of the absorption. Path length through exhaust ca. 1 m. Gauze Attenuator Retroreflector Air Flow Exhaust Plume Detector June 22 nd 2010 Laser 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

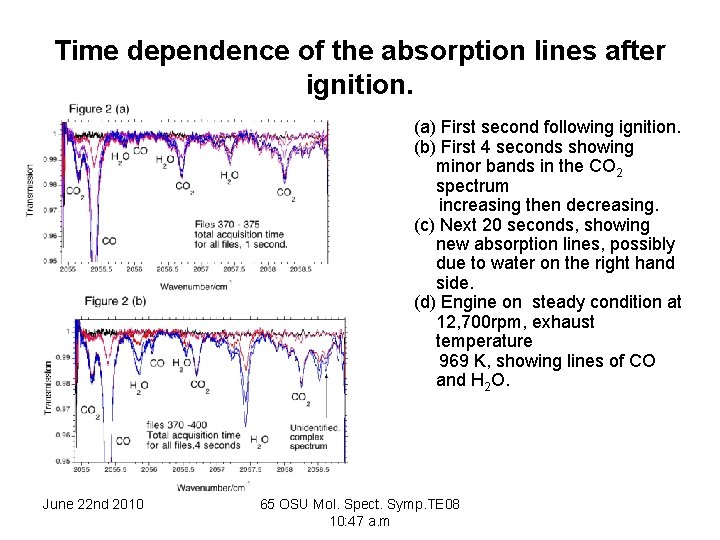
Time dependence of the absorption lines after ignition. (a) First second following ignition. (b) First 4 seconds showing minor bands in the CO 2 spectrum increasing then decreasing. (c) Next 20 seconds, showing new absorption lines, possibly due to water on the right hand side. (d) Engine on steady condition at 12, 700 rpm, exhaust temperature 969 K, showing lines of CO and H 2 O. June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

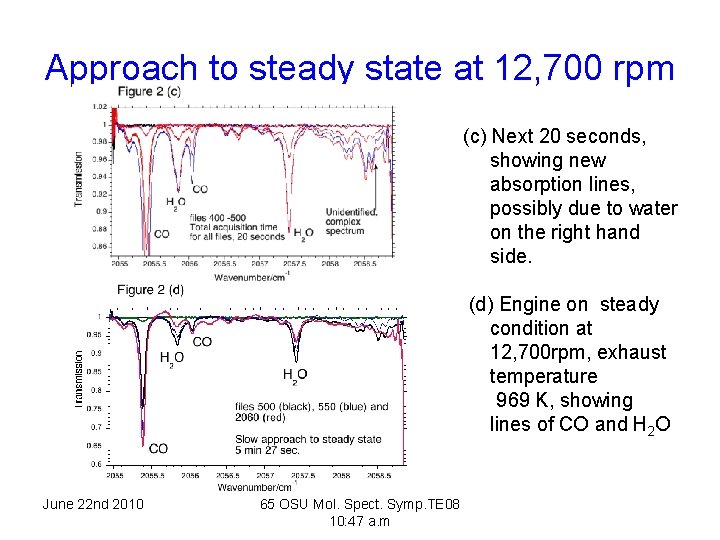
Approach to steady state at 12, 700 rpm (c) Next 20 seconds, showing new absorption lines, possibly due to water on the right hand side. (d) Engine on steady condition at 12, 700 rpm, exhaust temperature 969 K, showing lines of CO and H 2 O June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

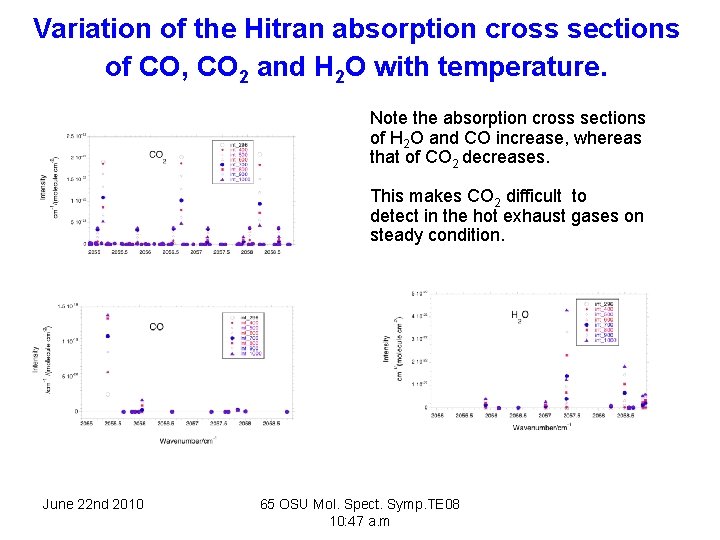
Variation of the Hitran absorption cross sections of CO, CO 2 and H 2 O with temperature. Note the absorption cross sections of H 2 O and CO increase, whereas that of CO 2 decreases. This makes CO 2 difficult to detect in the hot exhaust gases on steady condition. June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

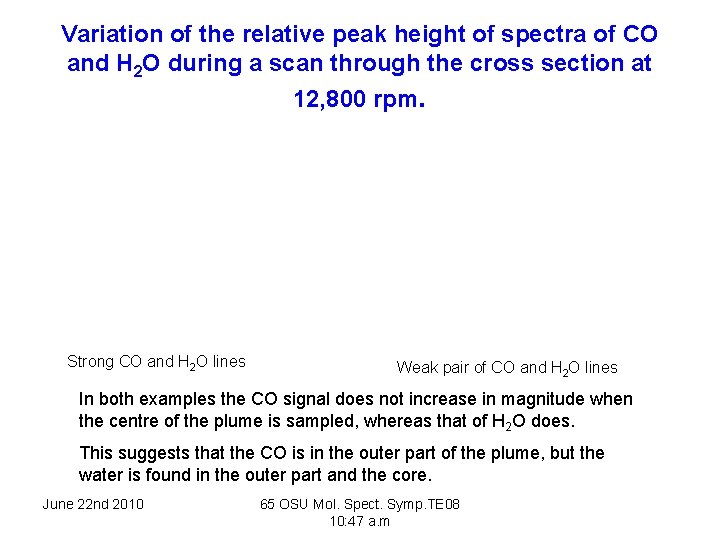
Variation of the relative peak height of spectra of CO and H 2 O during a scan through the cross section at 12, 800 rpm. Strong CO and H 2 O lines Weak pair of CO and H 2 O lines In both examples the CO signal does not increase in magnitude when the centre of the plume is sampled, whereas that of H 2 O does. This suggests that the CO is in the outer part of the plume, but the water is found in the outer part and the core. June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

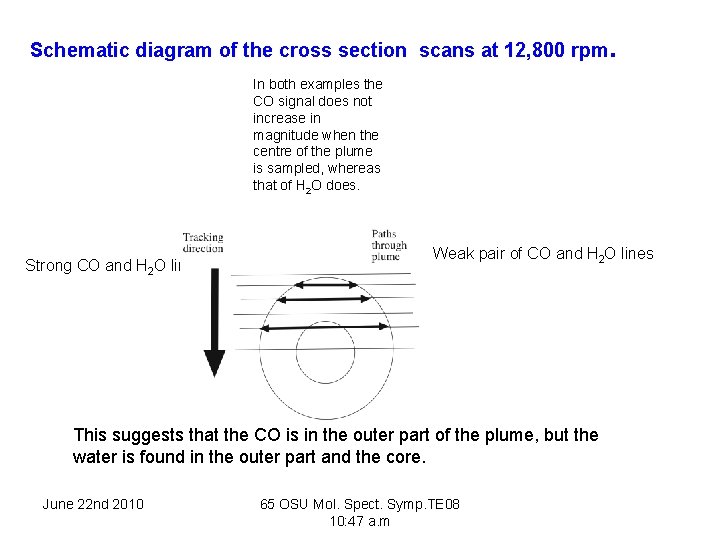
Schematic diagram of the cross section scans at 12, 800 rpm . In both examples the CO signal does not increase in magnitude when the centre of the plume is sampled, whereas that of H 2 O does. Strong CO and H 2 O lines Weak pair of CO and H 2 O lines This suggests that the CO is in the outer part of the plume, but the water is found in the outer part and the core. June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

Conclusions • Spectrometers based upon the intrapulse method are very useful for gas sensing • They can operate in a noisy environment such as that of a jet engine test cell such as that at RR Ansty. • The rapid acquisition of a single spectrum in 1 to 2 microseconds minimises the effects of acoustic noise • They have high sensitivity and good (for rapid measurement systems) frequency coverage so that several species may be measured simultaneously. June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m

Acknowledgements We are indebted NERC for the award of a COSMAS grant and to the EPSRC for an instrumentation grant, and for the award to K. G. Hay of a studentship through the Doctoral Training Fund GD is grateful to the Leverhulme Trust for the award of an Emeritus Followship during which this project was carried out. June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m
![References 1 Geoffrey Duxbury Infrared VibrationRotation Spectroscopy From Free Radicals To The Infrared Sky References [1] Geoffrey Duxbury, “Infrared Vibration-Rotation Spectroscopy: From Free Radicals To The Infrared Sky”,](https://slidetodoc.com/presentation_image/047496446bfb2653486b9e14961ee13d/image-15.jpg)
References [1] Geoffrey Duxbury, “Infrared Vibration-Rotation Spectroscopy: From Free Radicals To The Infrared Sky”, Wiley, 2000 [2] G. Duxbury, N. Langford, M. T. Mc. Culloch and S. Wright, “Quantum cascade Semiconductor nfrared and far-infrared lasers: from trace gas sensing to non-linear optics ”, Chem. Soc. Rev. , 34, 921 -934 (2005) [3] M. T. Mc. Culloch, N. Langford and G. Duxbury, ”Real-time trace-level detection of carbon dioxide and ethylene in car exhaust gases”. Appl. Optics 44, 2887 -2894 (2005) [4] A. Cheesman, J. A. Smith, M. N. R. Ashfold, N. Langford, S. Wright and G. Duxbury, “Application of a quantum cascade laser for time-resolved, in situ probing of CH 4/H 2 and C 2 H 2/H 2 gas mixtures during microwave plasma enhanced chemical vapor deposition of diamond”, J. Phys. Chem A 110, 2821 -2828 (2006) [5] K. G. Hay, S. Wright, G. Duxbury and N. Langford, “In flight measurements of ambient methane, nitrous oxide and water using a quantum cascade laser based spectrometer”, App. Phys. B 90, 329 -337 (2008) [6] J. Ma, A. Cheesman, M. N. R. Ashfold, K G. Hay, S. Wright, N Langford, G Duxbury, and Y. A. Mankelevich , “Quantum cascade laser investigation of CH 4 and C 2 H 2 interconversion in hydrocarbon/H 2 gas mixtures during microwave plasma enhanced chemical vapor deposition of diamond”, J. App. Phys. 106, 033305 (1 -15) (2009) [7] N. Tasinato, G. Duxbury, N. Langford and K G. Hay, “An investigation of collisional processes in a Dicke narrowed transition of water vapor in the 7. 8 m spectral region by frequency down-chirped quantum cascade laser spectroscopy, ” J. Chem Phys. 132, 044316 (2010) June 22 nd 2010 65 OSU Mol. Spect. Symp. TE 08 10: 47 a. m