Reading Chemical Formulas Review Element Symbols All elements

Reading Chemical Formulas
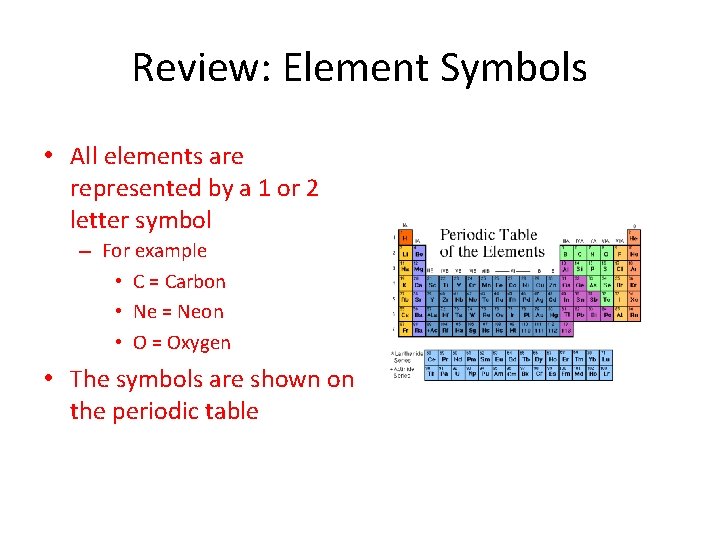
Review: Element Symbols • All elements are represented by a 1 or 2 letter symbol – For example • C = Carbon • Ne = Neon • O = Oxygen • The symbols are shown on the periodic table
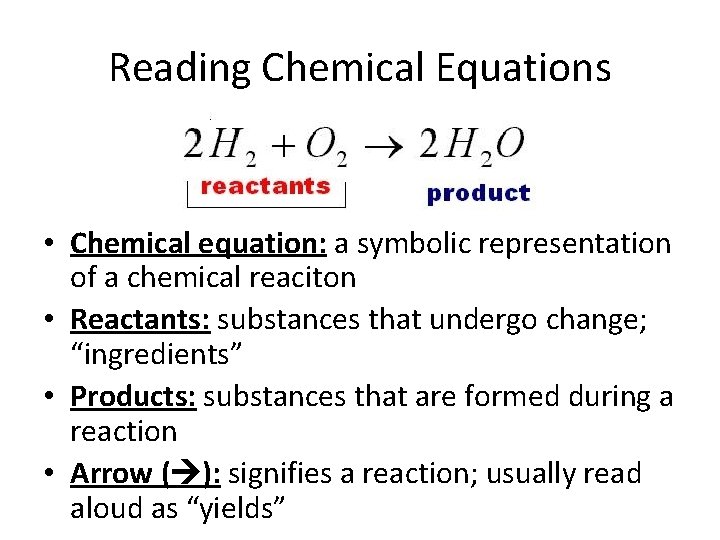
Reading Chemical Equations • Chemical equation: a symbolic representation of a chemical reaciton • Reactants: substances that undergo change; “ingredients” • Products: substances that are formed during a reaction • Arrow ( ): signifies a reaction; usually read aloud as “yields”
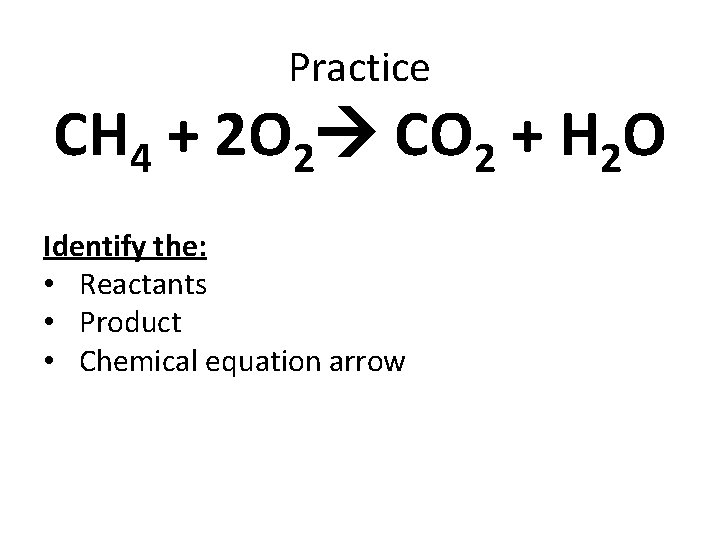
Practice CH 4 + 2 O 2 CO 2 + H 2 O Identify the: • Reactants • Product • Chemical equation arrow
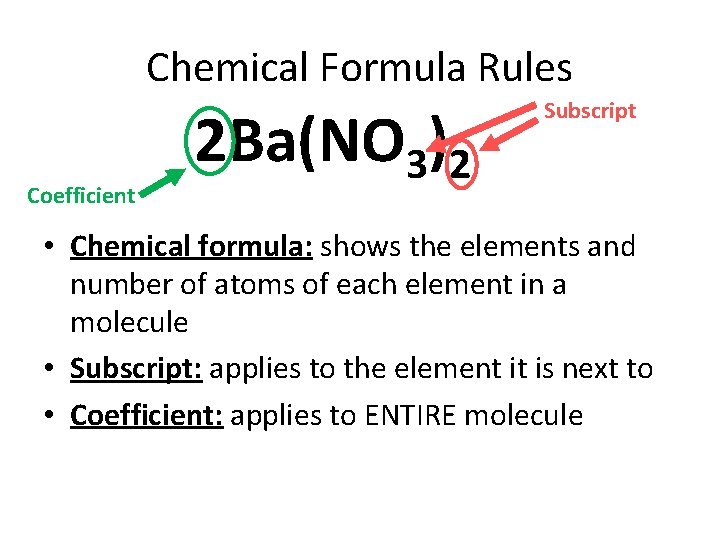
Chemical Formula Rules Coefficient 2 Ba(NO 3)2 Subscript • Chemical formula: shows the elements and number of atoms of each element in a molecule • Subscript: applies to the element it is next to • Coefficient: applies to ENTIRE molecule
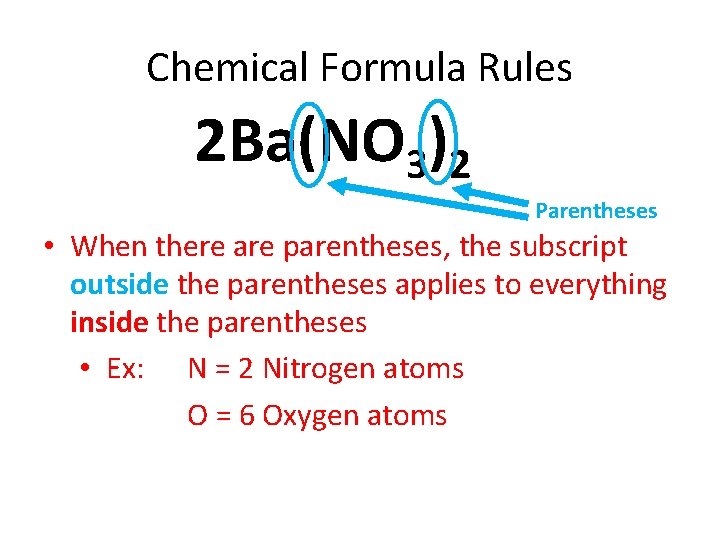
Chemical Formula Rules 2 Ba(NO 3)2 Parentheses • When there are parentheses, the subscript outside the parentheses applies to everything inside the parentheses • Ex: N = 2 Nitrogen atoms O = 6 Oxygen atoms
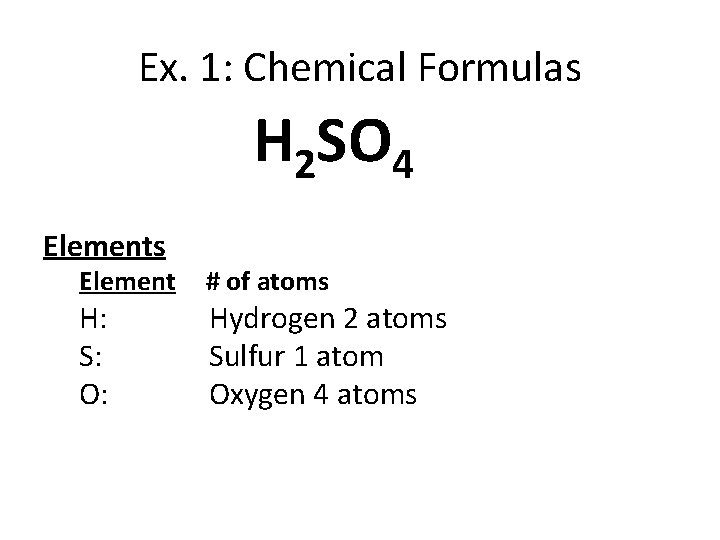
Ex. 1: Chemical Formulas H 2 SO 4 Elements Element H: S: O: # of atoms Hydrogen 2 atoms Sulfur 1 atom Oxygen 4 atoms
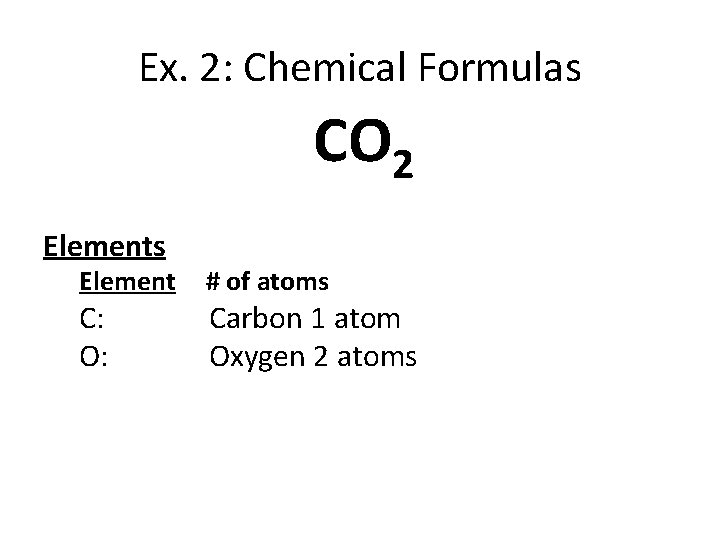
Ex. 2: Chemical Formulas CO 2 Elements Element C: O: # of atoms Carbon 1 atom Oxygen 2 atoms
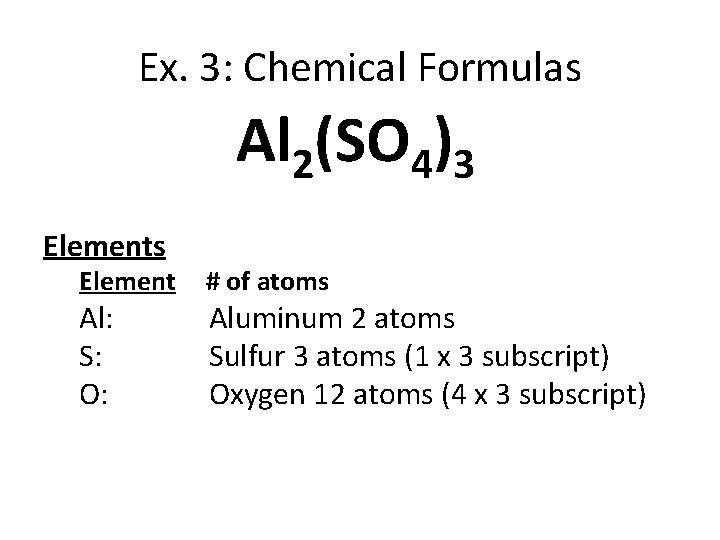
Ex. 3: Chemical Formulas Al 2(SO 4)3 Elements Element Al: S: O: # of atoms Aluminum 2 atoms Sulfur 3 atoms (1 x 3 subscript) Oxygen 12 atoms (4 x 3 subscript)

Practice Write out the #of elements: A. Al. PO 4 B. S 2 C. Al 2(SO 3)3
- Slides: 10