Reactions of Hydrocarbons Combustion Addition Substitution Esterification Fermentation

Reactions of Hydrocarbons • • • Combustion Addition Substitution Esterification Fermentation • Saponification • Addition Polymerization • Condensation Polymerization

• Reaction with oxygen or burning. • O 2 is on the reactant side. • Complete combustion of HC produces CO 2 & H 2 O. Combustion
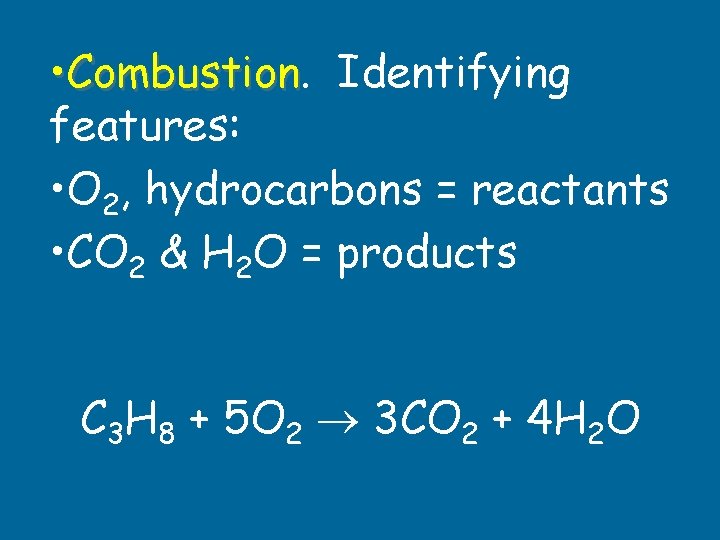
• Combustion Identifying features: • O 2, hydrocarbons = reactants • CO 2 & H 2 O = products C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O

• Start with an alkene or alkyne. • Add atoms across the unsaturated bond. • Commonly: hydrogenation or halogenation. Addition
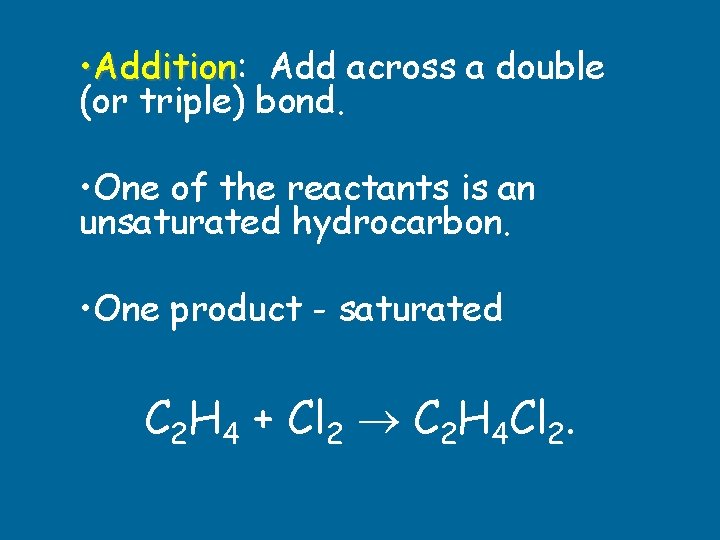
• Addition: Addition Add across a double (or triple) bond. • One of the reactants is an unsaturated hydrocarbon. • One product - saturated C 2 H 4 + Cl 2 C 2 H 4 Cl 2.
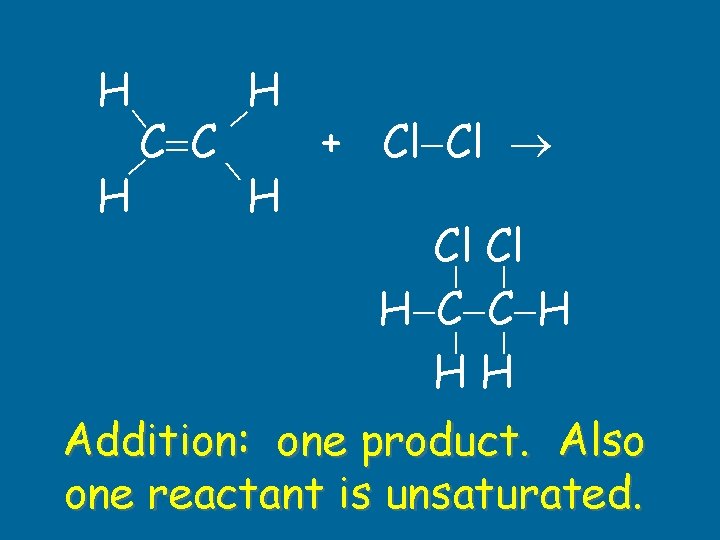
H C C + Cl Cl H H Cl Cl H C C H HH H Addition: one product. Also one reactant is unsaturated.
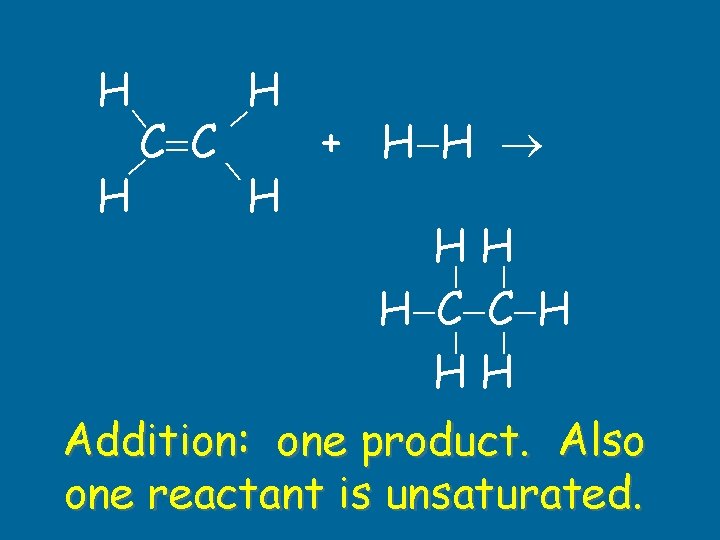
H C C + H H HH H C C H HH H Addition: one product. Also one reactant is unsaturated.

• Substitution: Substitution Replace 1 or more H’s in a saturated hydrocarbon with another atom or group of atoms. • One of the reactants is a saturated hydrocarbon. • Two products C 2 H 6 + Cl 2 C 2 H 5 Cl + HCl
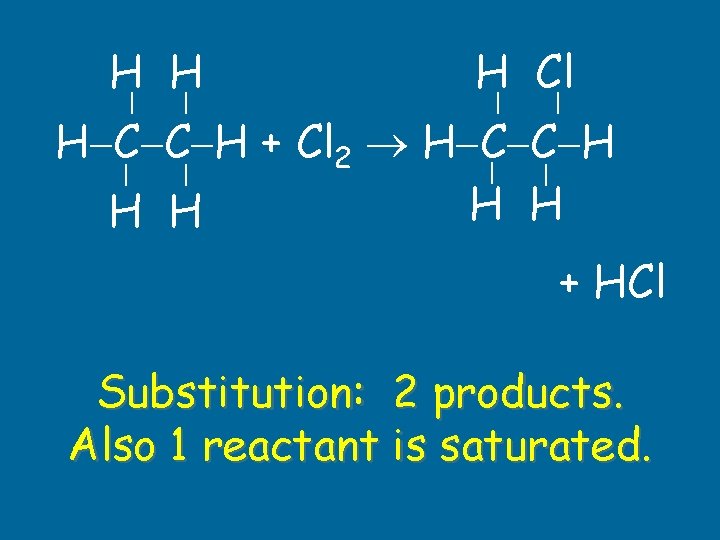
H Cl H H H C C H + Cl 2 H C C H H H + HCl Substitution: 2 products. Also 1 reactant is saturated.
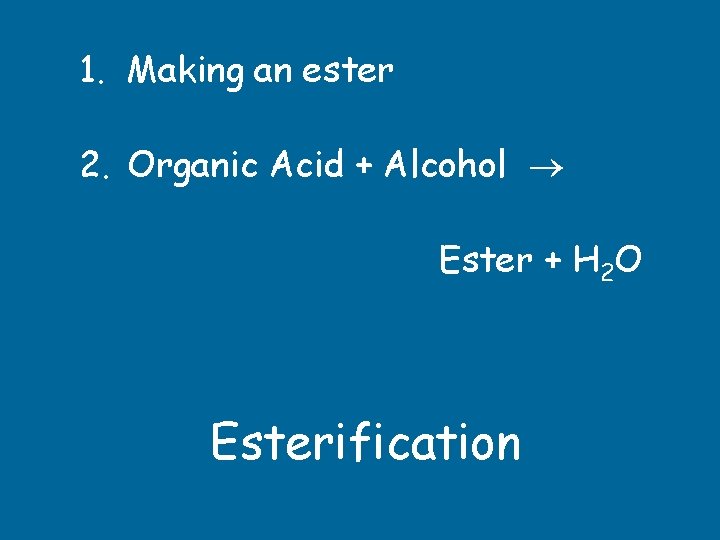
1. Making an ester 2. Organic Acid + Alcohol Ester + H 2 O Esterification
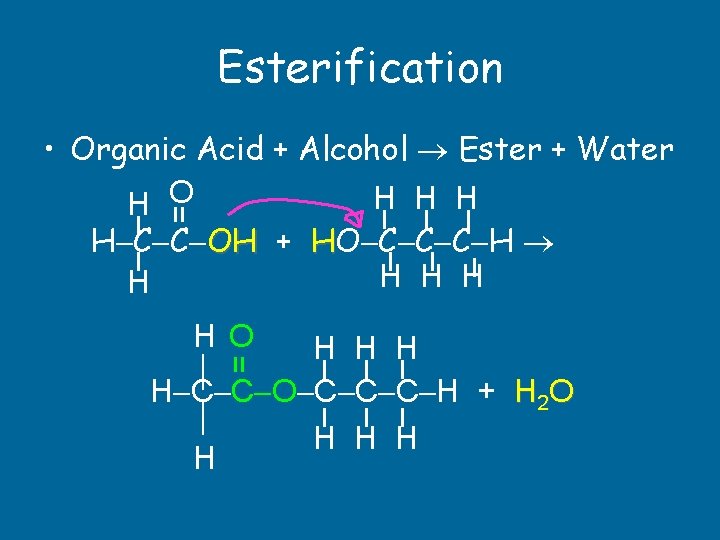
Esterification = • Organic Acid + Alcohol Ester + Water H H O H C C OH + HO C C C H H H = H O H H H H C C O C C C H + H 2 O H H
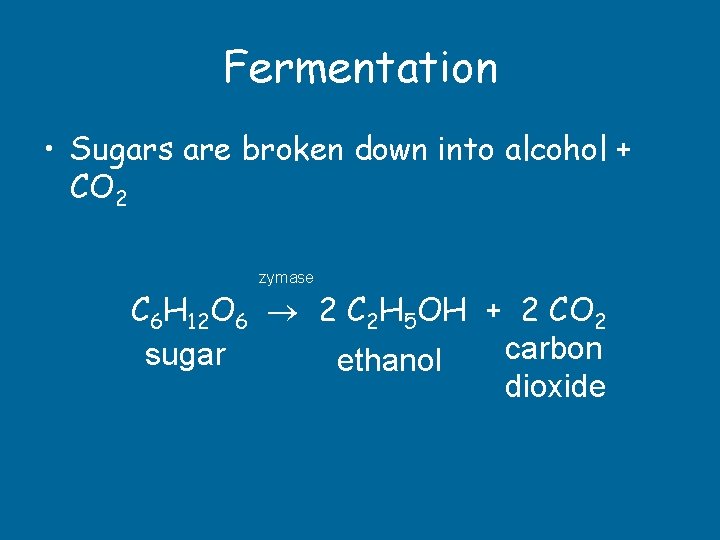
Fermentation • Sugars are broken down into alcohol + CO 2 zymase C 6 H 12 O 6 2 C 2 H 5 OH + 2 CO 2 carbon sugar ethanol dioxide

Saponification • Making Soap • Fat + Base Glycerol + Soap
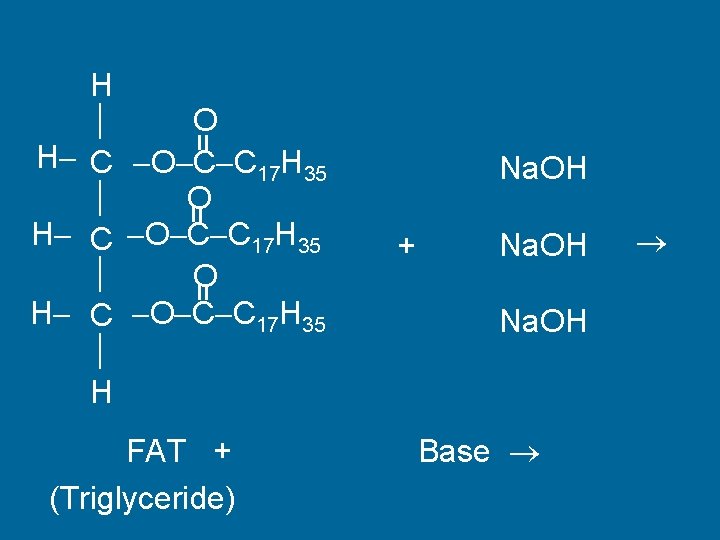
= H O H C O C C 17 H 35 H = Na. OH = + FAT + (Triglyceride) Na. OH Base
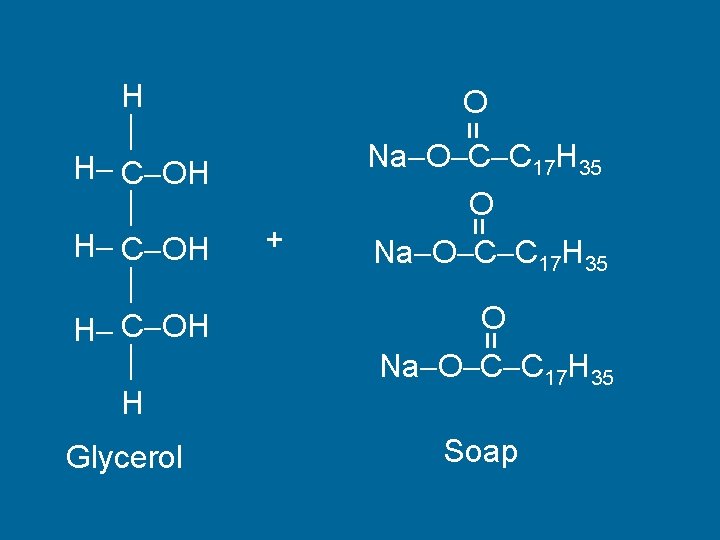
Glycerol = O O Na O C C 17 H 35 = + Na O C C 17 H 35 O Na O C C 17 H 35 = H H C OH H Soap

1. Reaction leading to formation of polymers Polymerization
Polymerization Reactions • Addition: Addition start with unsaturated monomers. Everything in reactants goes into polymer. Only 1 product, saturated. • Condensation: Condensation monomers containing 2 functional groups combine with the loss of a small by-product, usually H 2 O.
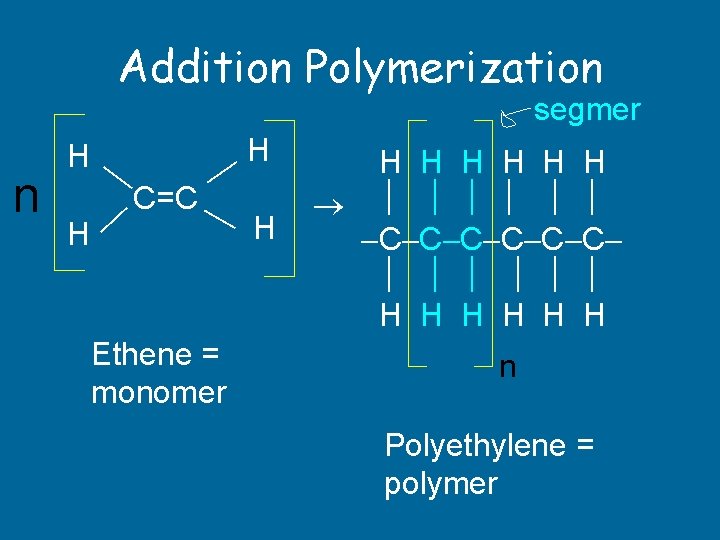
Addition Polymerization segmer C=C H n H H Ethene = monomer H H H H C C C H H H n Polyethylene = polymer
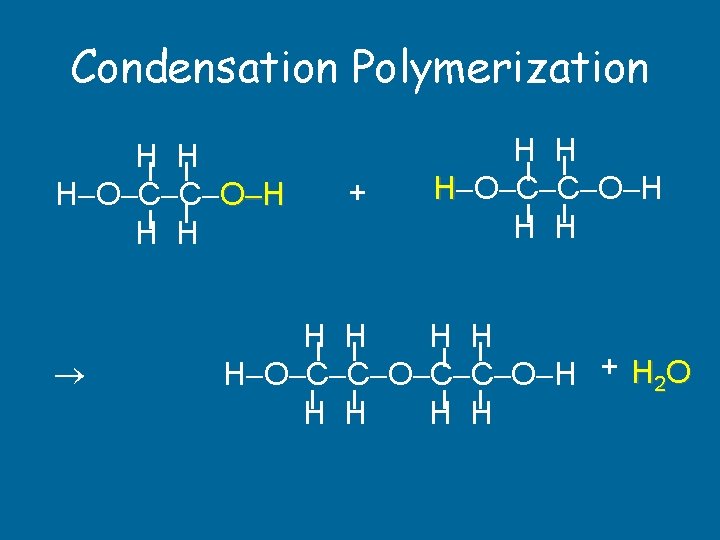
Condensation Polymerization H H H O C C O H H H + H H H O C C O H + H 2 O H H
Condensation Polymerization • Split out a small molecule such as H 2 O. • Monomer has to have a functional group at each end.
- Slides: 20