Reactions of Hydrocarbons Combustion 2 CH 3 CH
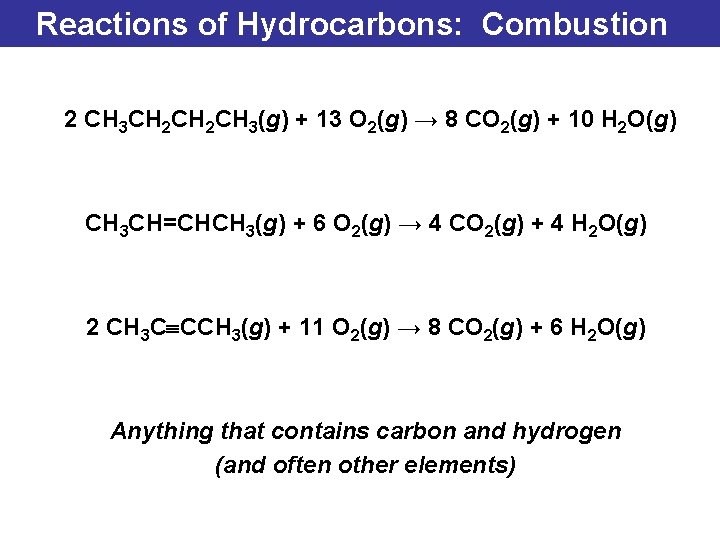
Reactions of Hydrocarbons: Combustion 2 CH 3 CH 2 CH 3(g) + 13 O 2(g) → 8 CO 2(g) + 10 H 2 O(g) CH 3 CH=CHCH 3(g) + 6 O 2(g) → 4 CO 2(g) + 4 H 2 O(g) 2 CH 3 C CCH 3(g) + 11 O 2(g) → 8 CO 2(g) + 6 H 2 O(g) Anything that contains carbon and hydrogen (and often other elements)
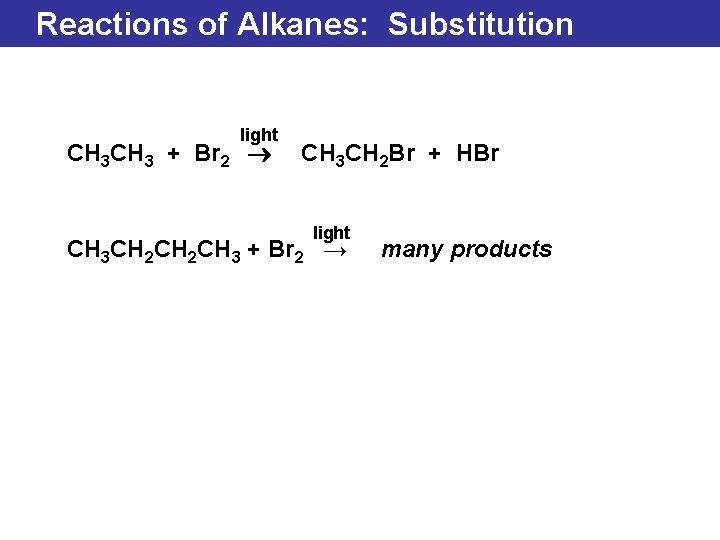
Reactions of Alkanes: Substitution light CH 3 + Br 2 ® CH 3 CH 2 Br + HBr light CH 3 CH 2 CH 3 + Br 2 → many products
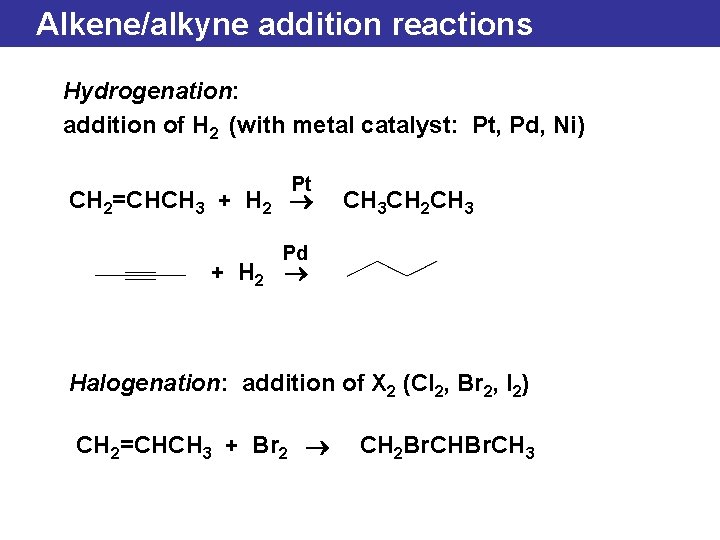
Alkene/alkyne addition reactions Hydrogenation: addition of H 2 (with metal catalyst: Pt, Pd, Ni) Pt CH 2=CHCH 3 + H 2 ® CH 3 CH 2 CH 3 Pd + H 2 ® Halogenation: addition of X 2 (Cl 2, Br 2, I 2) CH 2=CHCH 3 + Br 2 ® CH 2 Br. CH 3
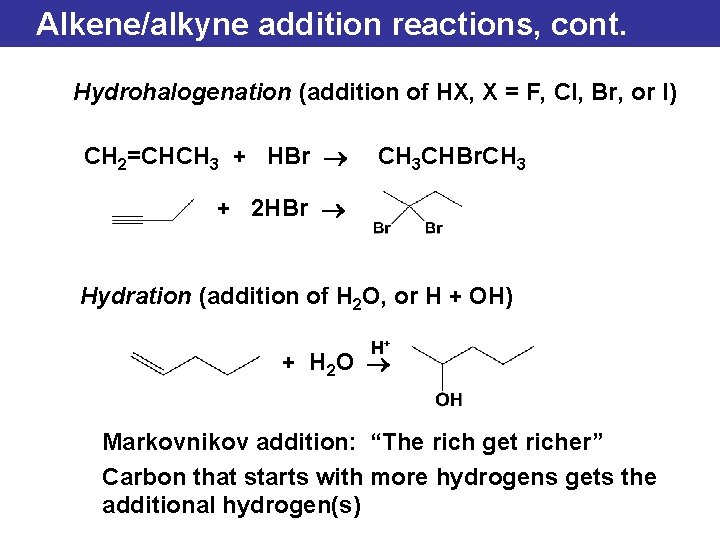
Alkene/alkyne addition reactions, cont. Hydrohalogenation (addition of HX, X = F, Cl, Br, or I) CH 2=CHCH 3 + HBr ® CH 3 CHBr. CH 3 + 2 HBr ® Hydration (addition of H 2 O, or H + OH) H+ + H 2 O ® Markovnikov addition: “The rich get richer” Carbon that starts with more hydrogens gets the additional hydrogen(s)
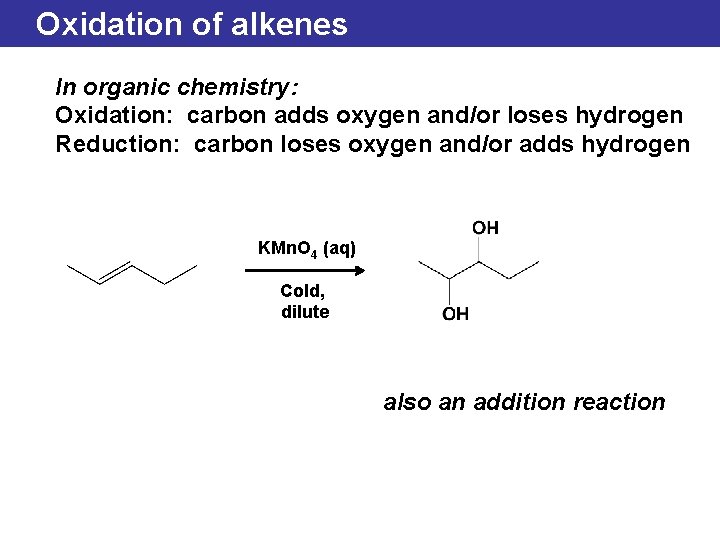
Oxidation of alkenes In organic chemistry: Oxidation: carbon adds oxygen and/or loses hydrogen Reduction: carbon loses oxygen and/or adds hydrogen KMn. O 4 (aq) Cold, dilute also an addition reaction
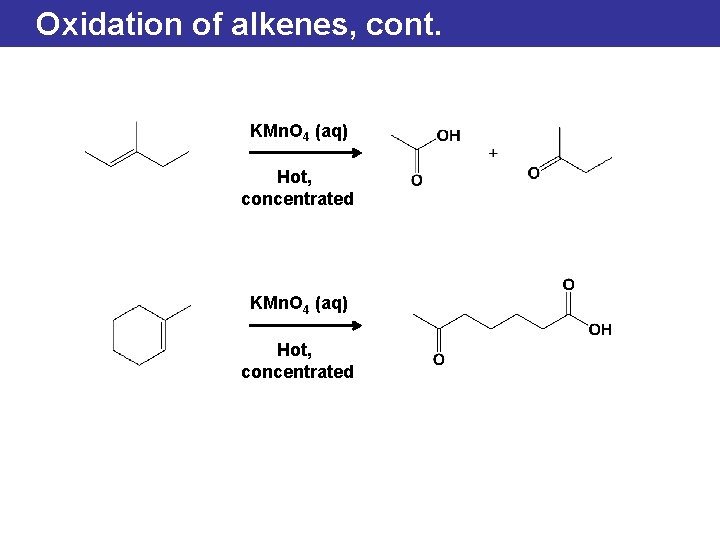
Oxidation of alkenes, cont. KMn. O 4 (aq) Hot, concentrated
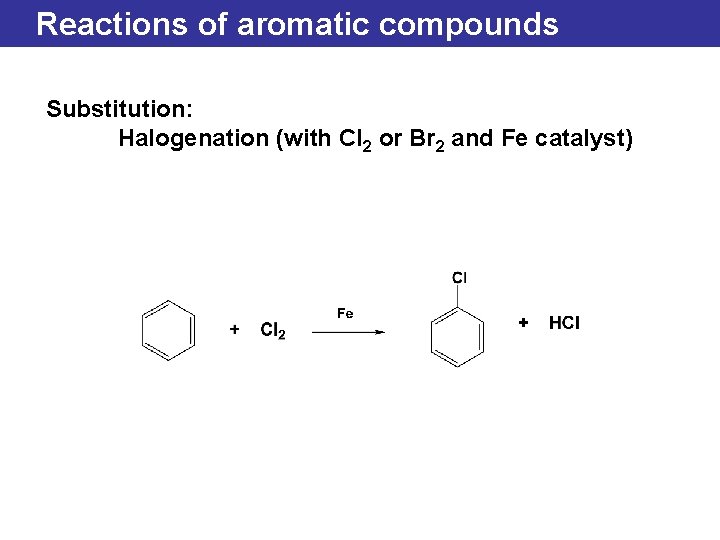
Reactions of aromatic compounds Substitution: Halogenation (with Cl 2 or Br 2 and Fe catalyst)
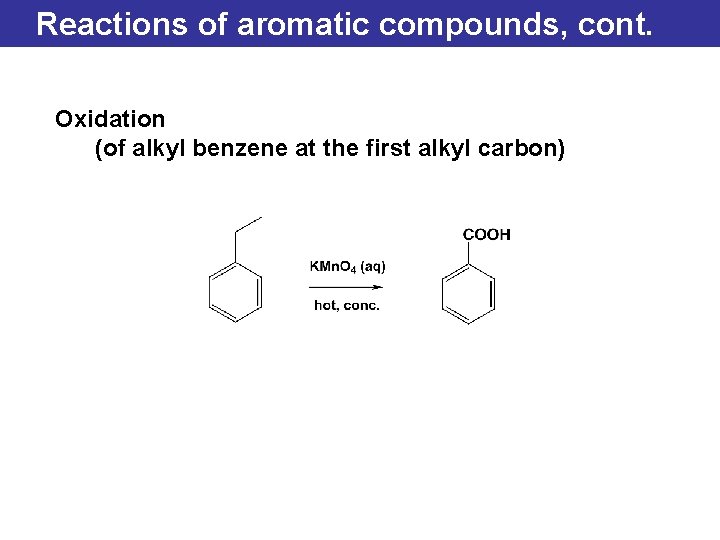
Reactions of aromatic compounds, cont. Oxidation (of alkyl benzene at the first alkyl carbon)
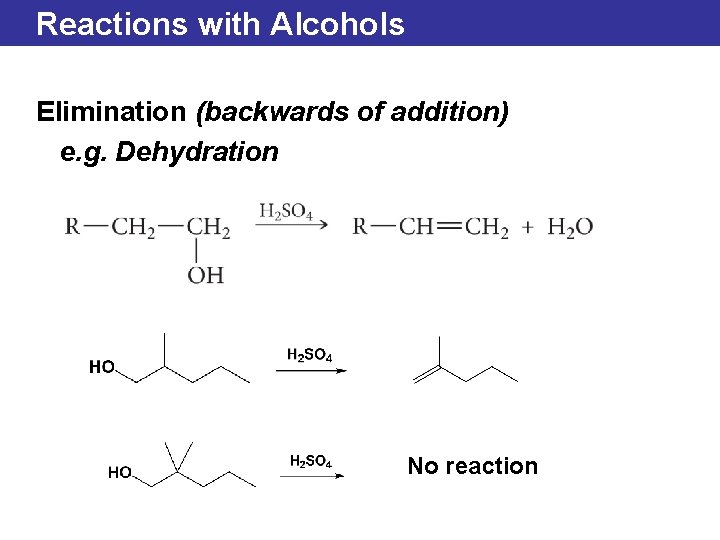
Reactions with Alcohols Elimination (backwards of addition) e. g. Dehydration No reaction
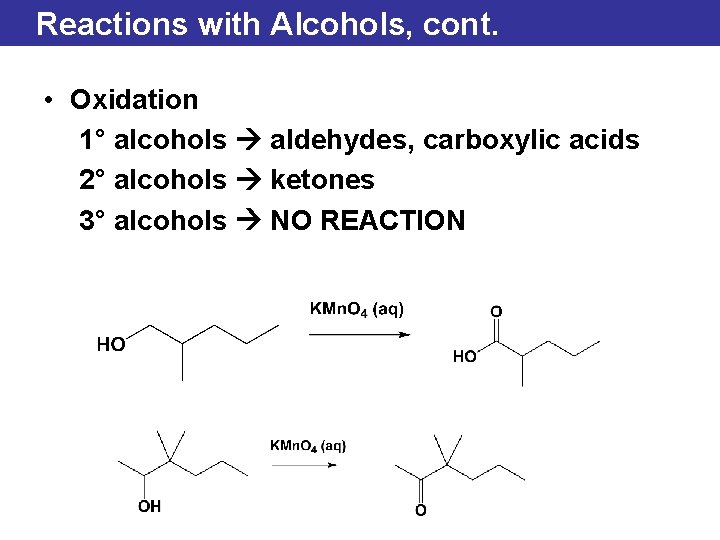
Reactions with Alcohols, cont. • Oxidation 1° alcohols aldehydes, carboxylic acids 2° alcohols ketones 3° alcohols NO REACTION
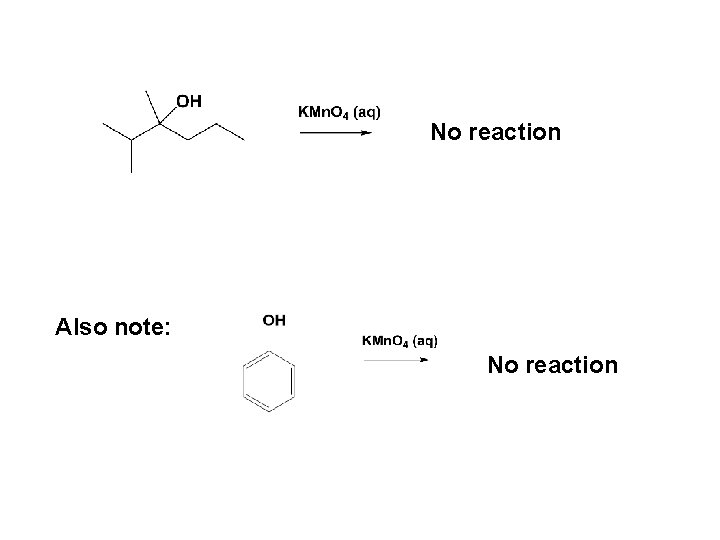
No reaction Also note: No reaction
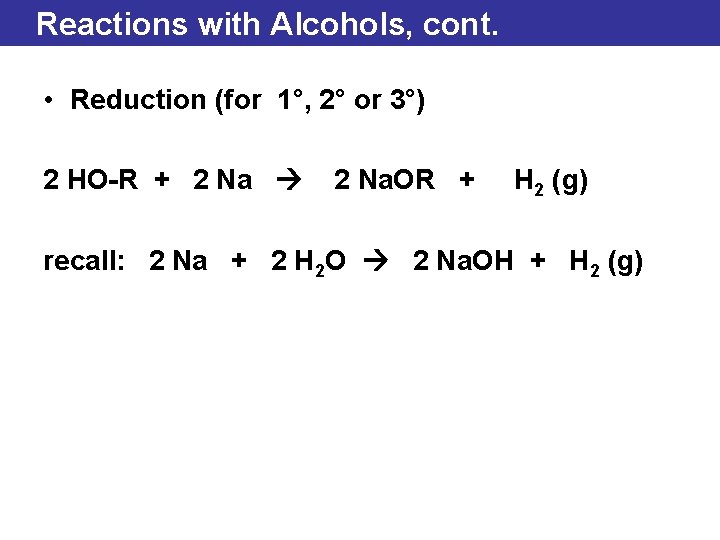
Reactions with Alcohols, cont. • Reduction (for 1°, 2° or 3°) 2 HO-R + 2 Na. OR + H 2 (g) recall: 2 Na + 2 H 2 O 2 Na. OH + H 2 (g)
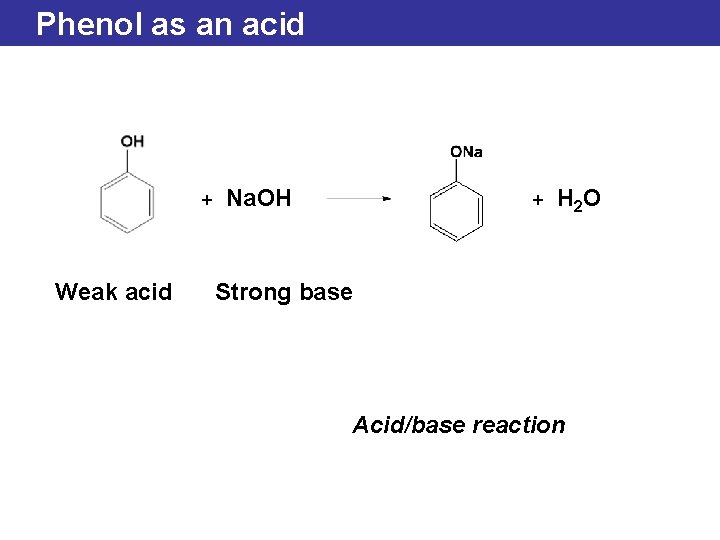
Phenol as an acid + Weak acid Na. OH + H 2 O Strong base Acid/base reaction
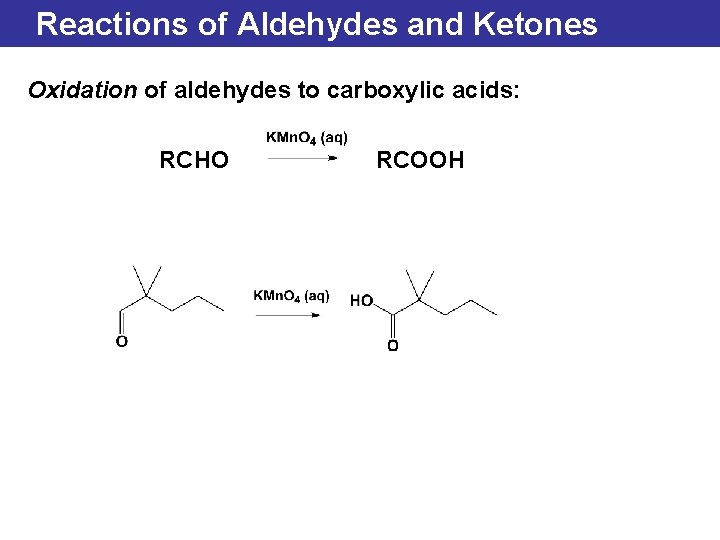
Reactions of Aldehydes and Ketones Oxidation of aldehydes to carboxylic acids: RCHO RCOOH
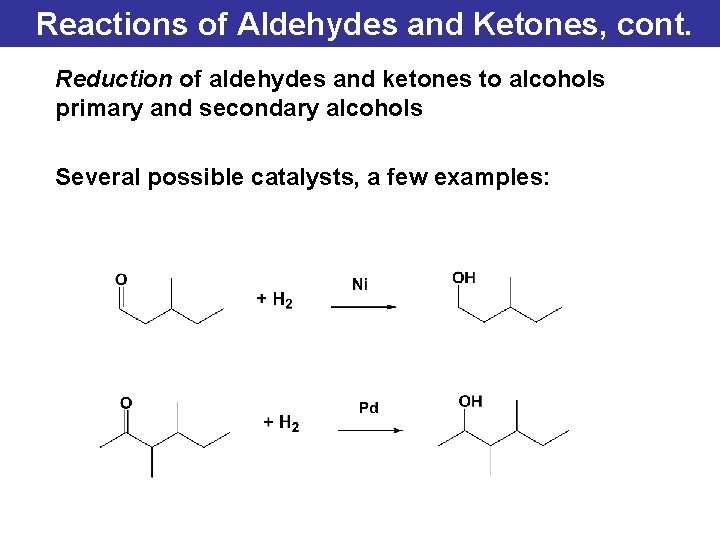
Reactions of Aldehydes and Ketones, cont. Reduction of aldehydes and ketones to alcohols primary and secondary alcohols Several possible catalysts, a few examples:
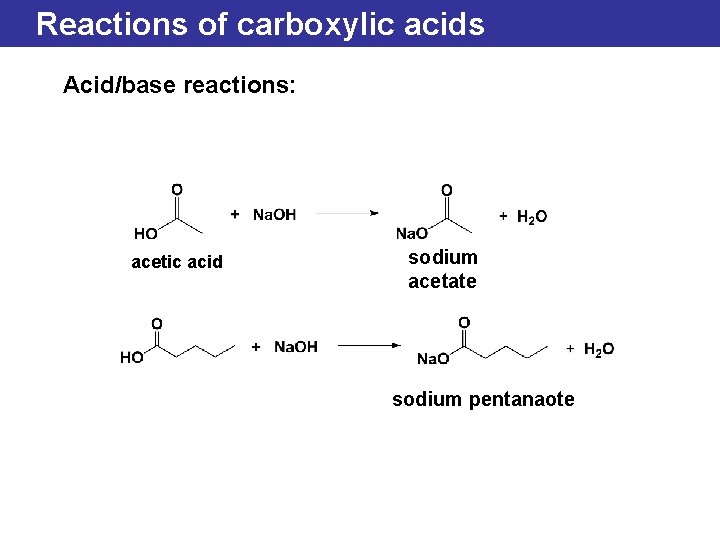
Reactions of carboxylic acids Acid/base reactions: acetic acid sodium acetate sodium pentanaote
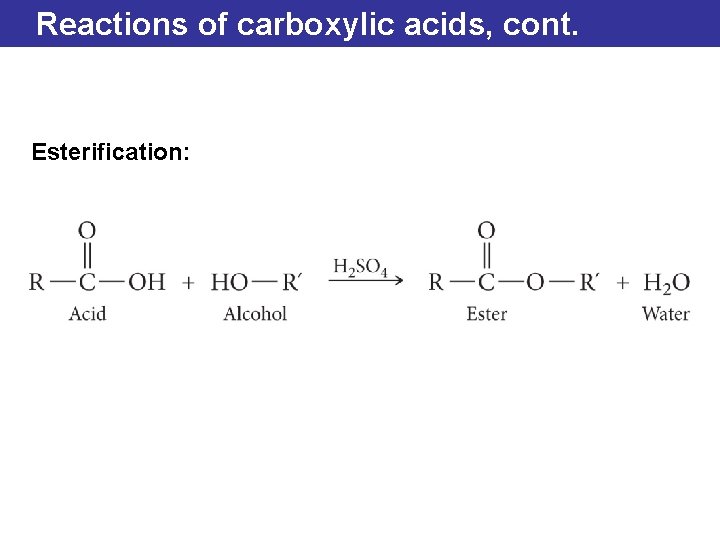
Reactions of carboxylic acids, cont. Esterification:
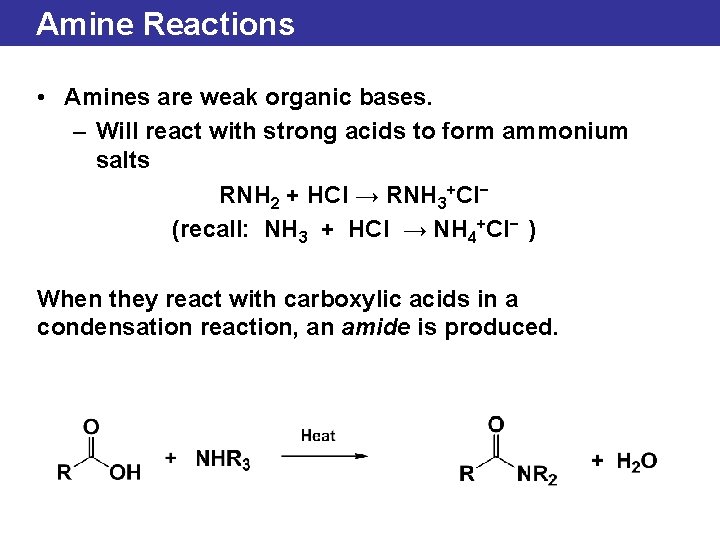
Amine Reactions • Amines are weak organic bases. – Will react with strong acids to form ammonium salts RNH 2 + HCl → RNH 3+Cl− (recall: NH 3 + HCl → NH 4+Cl− ) When they react with carboxylic acids in a condensation reaction, an amide is produced.
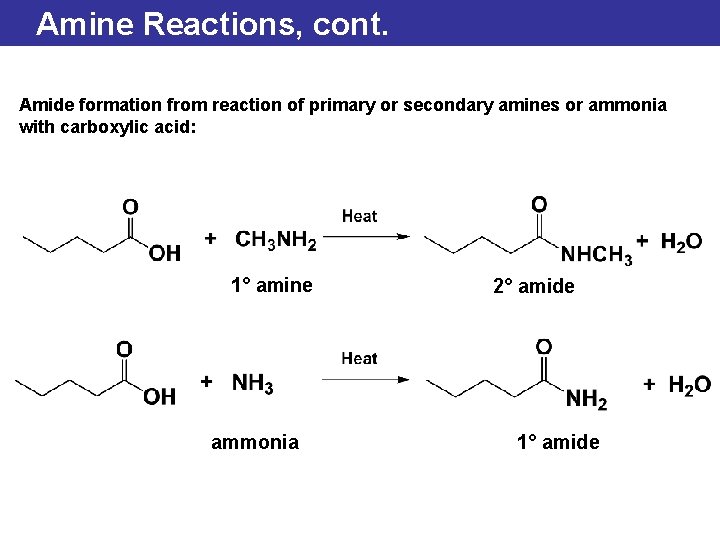
Amine Reactions, cont. Amide formation from reaction of primary or secondary amines or ammonia with carboxylic acid: 1° amine ammonia 2° amide 1° amide
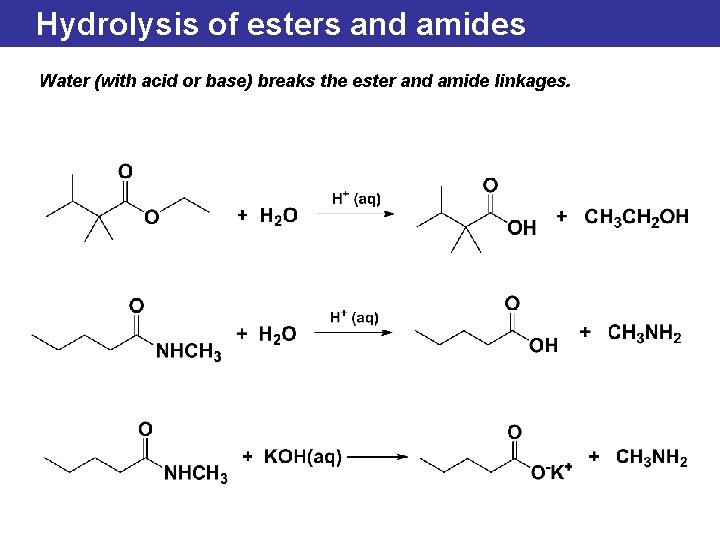
Hydrolysis of esters and amides Water (with acid or base) breaks the ester and amide linkages.
- Slides: 20