Reactions of aldehydes and ketones oxidation reduction nucleophilic








- Slides: 51

Reactions of aldehydes and ketones: oxidation reduction nucleophilic addition 1) Aldehydes are easily oxidized, ketones are not. 2) Aldehydes are more reactive in nucleophilic additions than ketones.

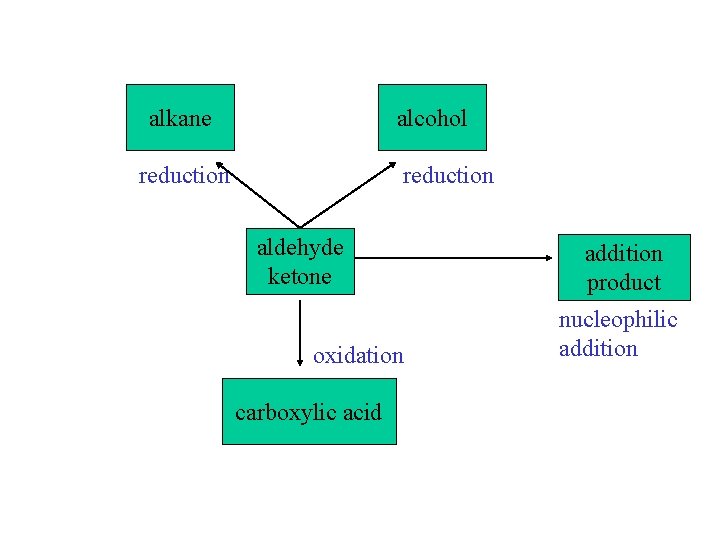
alkane alcohol reduction aldehyde ketone oxidation carboxylic acid addition product nucleophilic addition

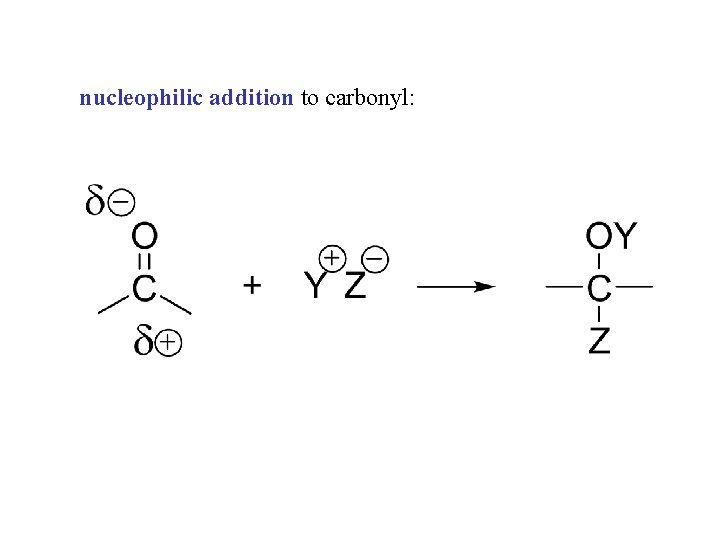
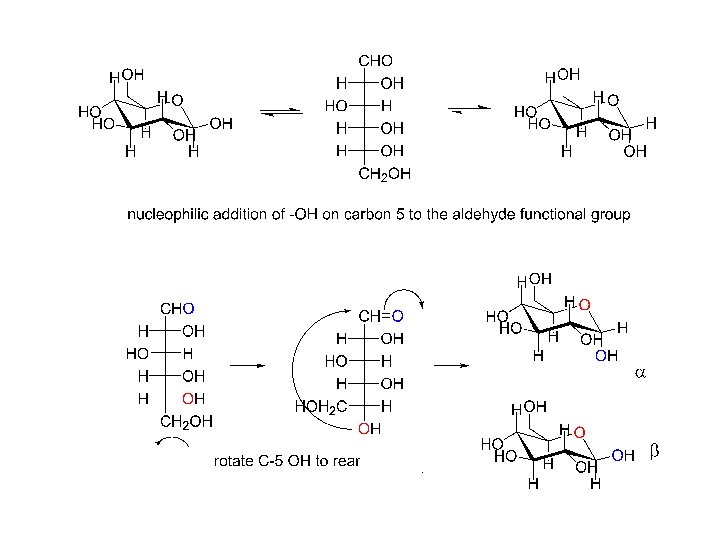
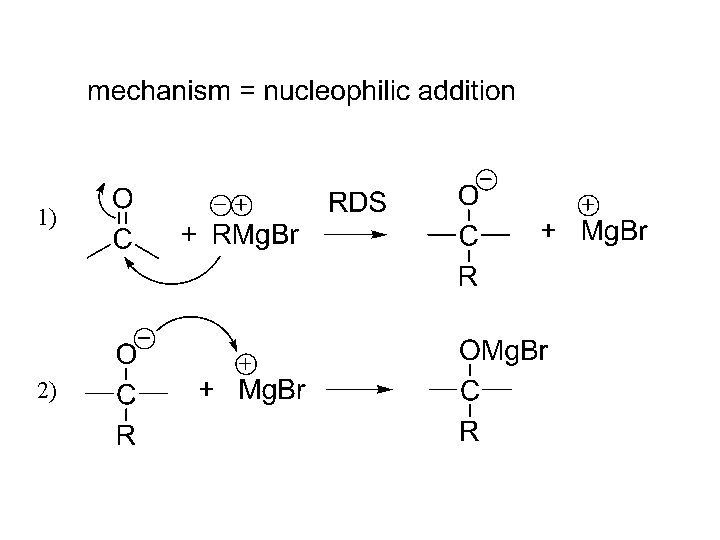
nucleophilic addition to carbonyl:

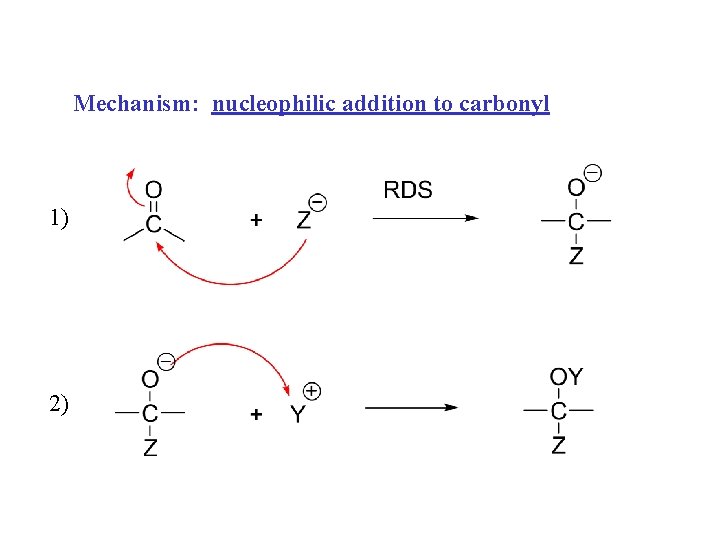
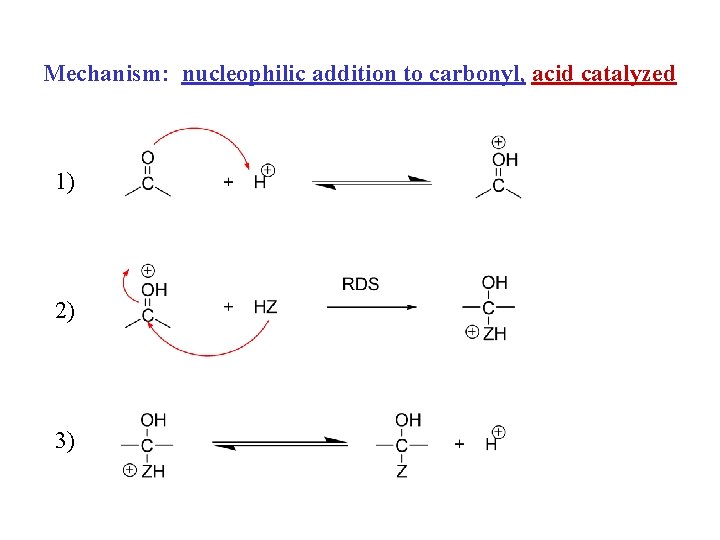
Mechanism: nucleophilic addition to carbonyl 1) 2)

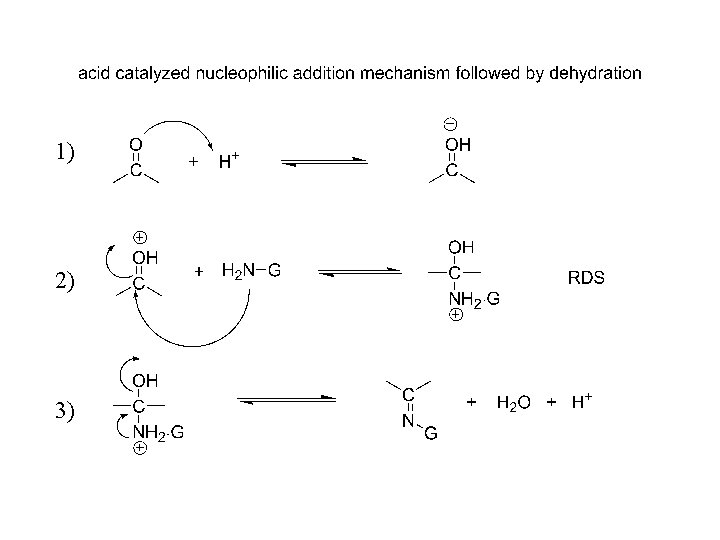
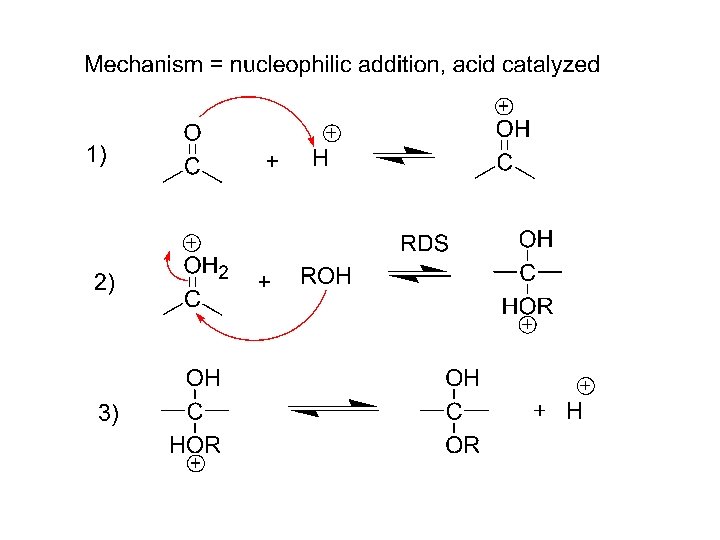
Mechanism: nucleophilic addition to carbonyl, acid catalyzed 1) 2) 3)

Aldehydes & ketones, reactions: 1) Oxidation 2) Reduction 3) Addition of cyanide 4) Addition of derivatives of ammonia 5) Addition of alcohols 6) Cannizzaro reaction 7) Addition of Grignard reagents 8) 8) (Alpha-halogenation of ketones) 9) 9) (Addition of carbanions)

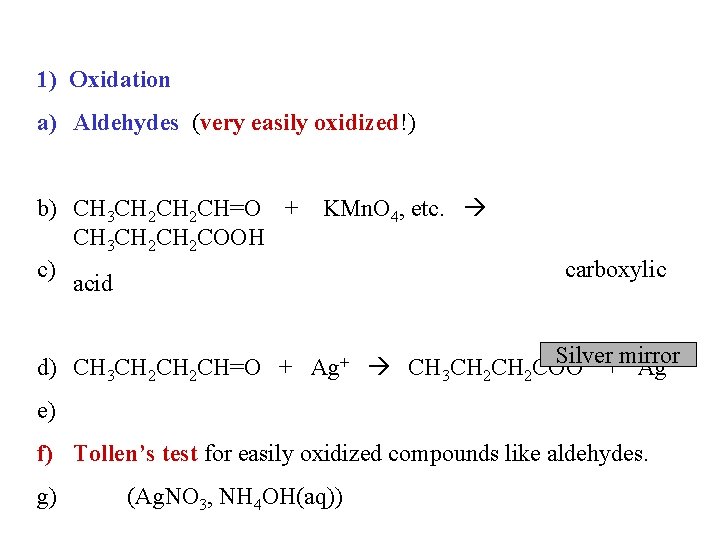
1) Oxidation a) Aldehydes (very easily oxidized!) b) CH 3 CH 2 CH=O + KMn. O 4, etc. CH 3 CH 2 COOH c) acid carboxylic Silver - +mirror d) CH 3 CH 2 CH=O + Ag+ CH 3 CH 2 COO Ag e) f) Tollen’s test for easily oxidized compounds like aldehydes. g) (Ag. NO 3, NH 4 OH(aq))

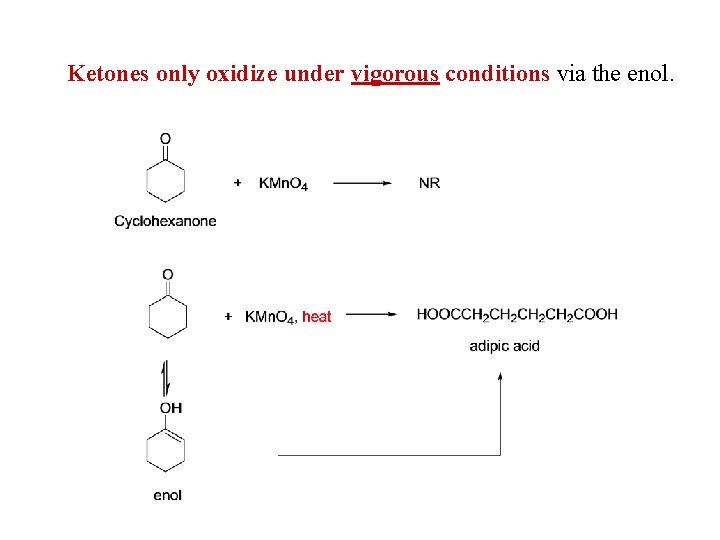
Ketones only oxidize under vigorous conditions via the enol.

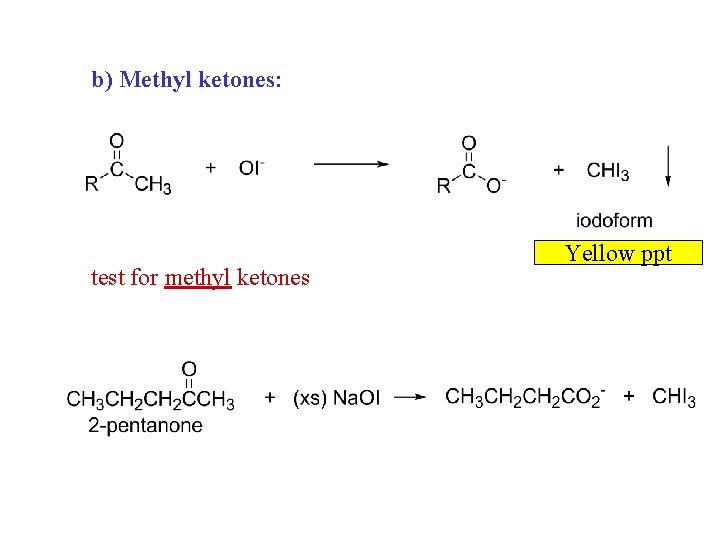
b) Methyl ketones: test for methyl ketones Yellow ppt

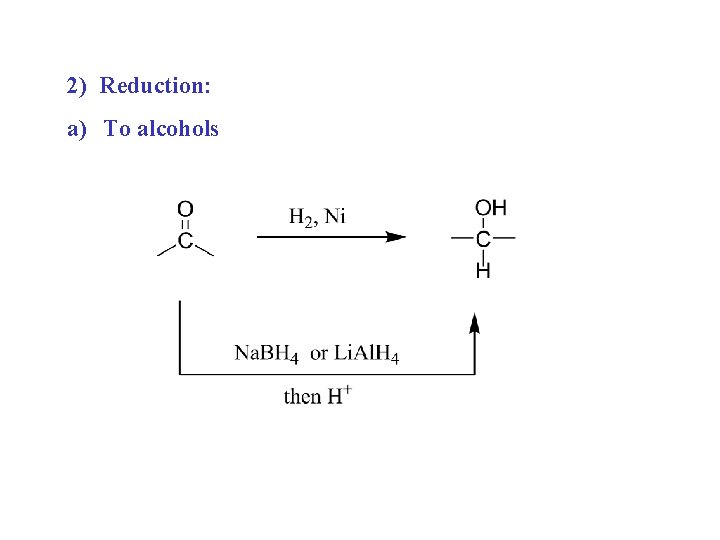
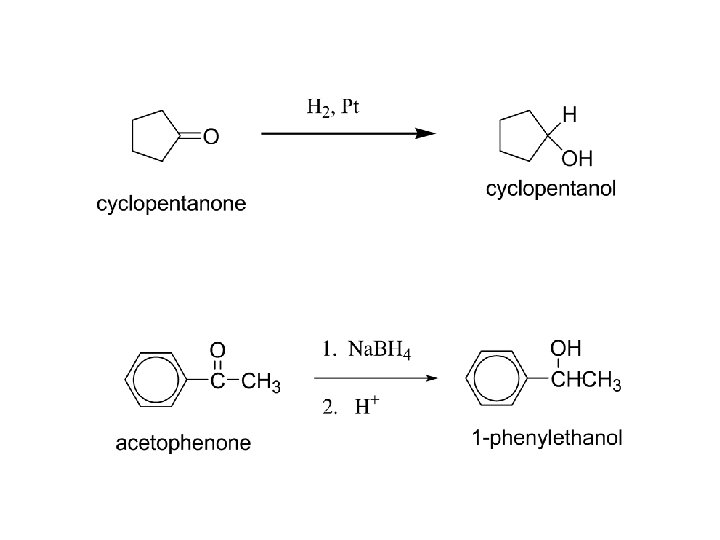
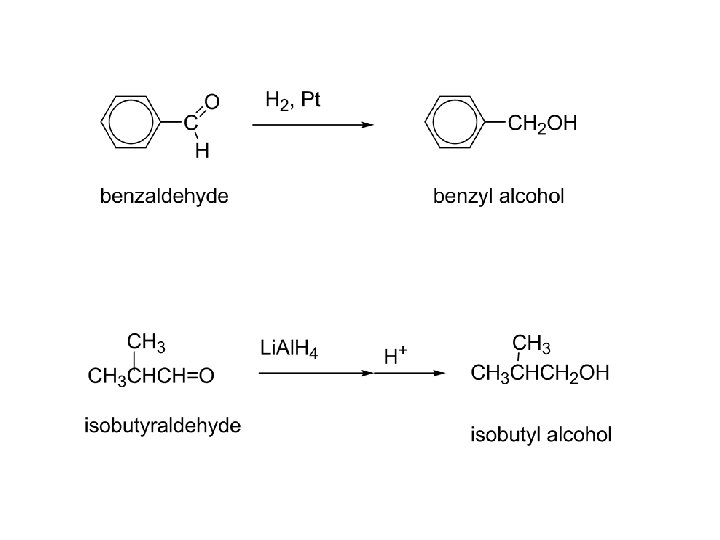
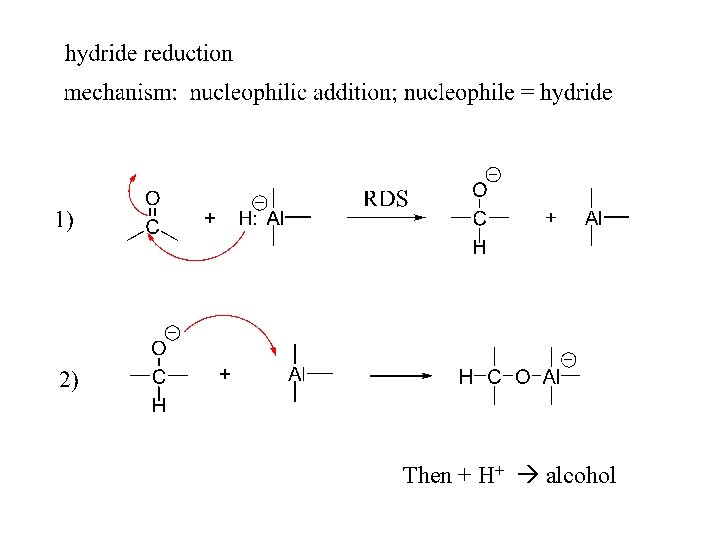
2) Reduction: a) To alcohols



Then + H+ alcohol

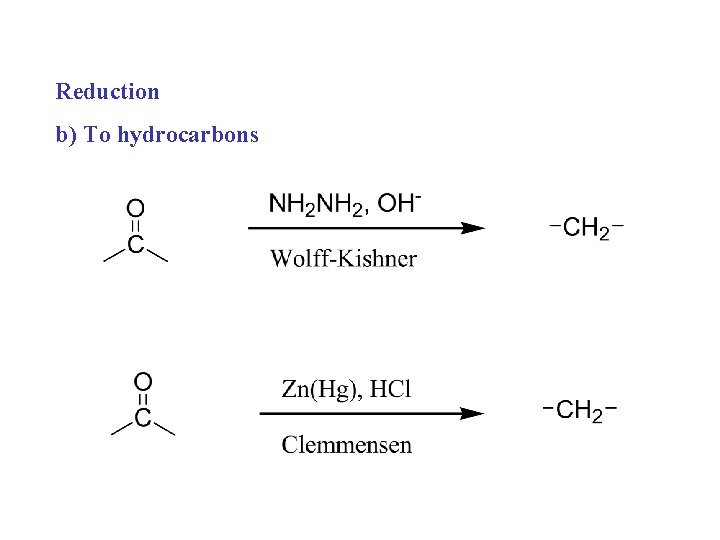
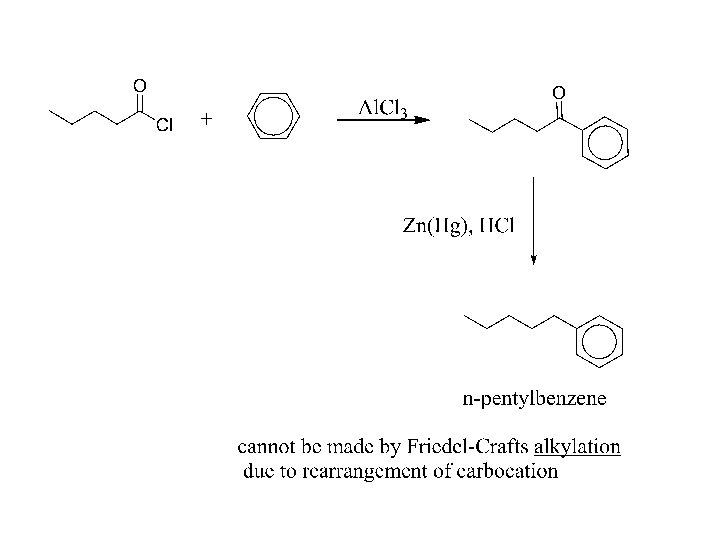
Reduction b) To hydrocarbons


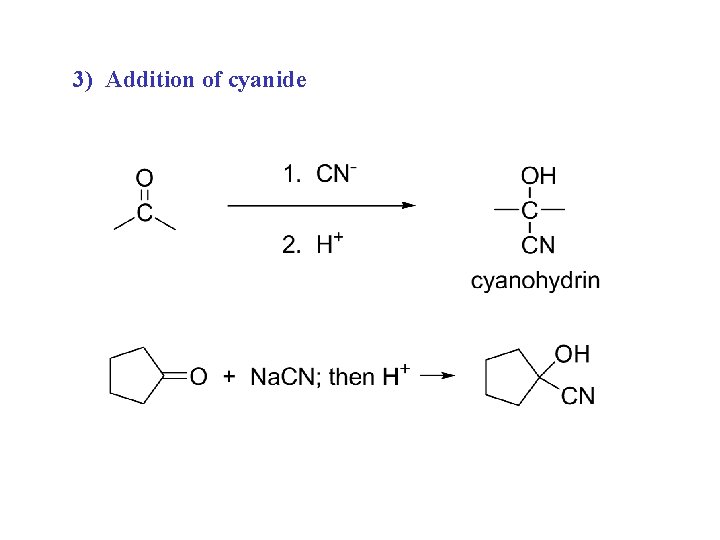
3) Addition of cyanide

1) 2)

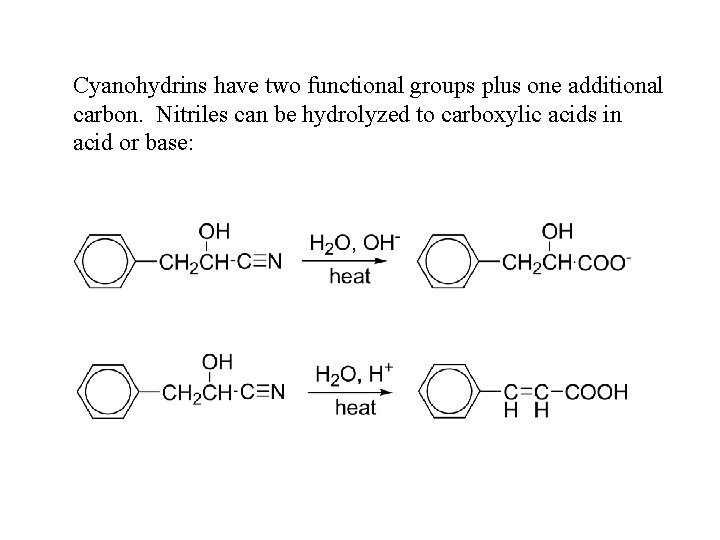
Cyanohydrins have two functional groups plus one additional carbon. Nitriles can be hydrolyzed to carboxylic acids in acid or base:

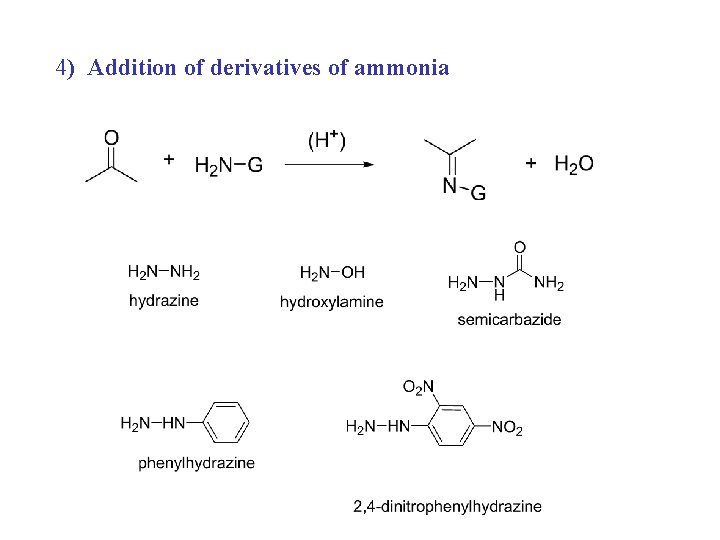
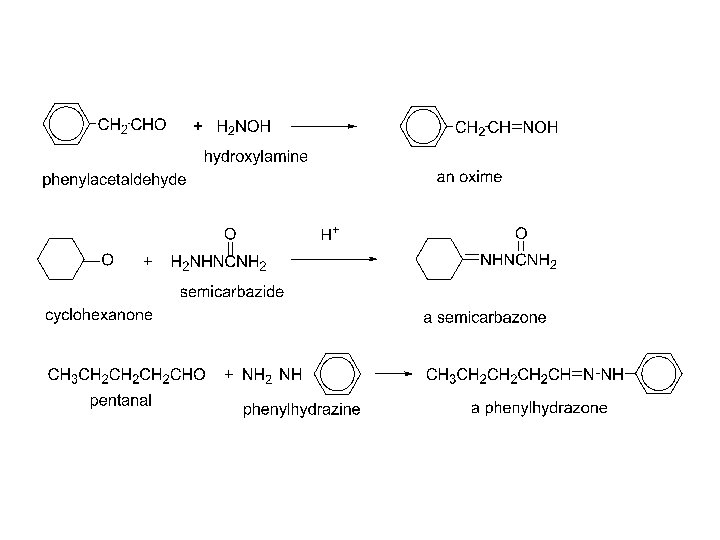
4) Addition of derivatives of ammonia

1) 2) 3)


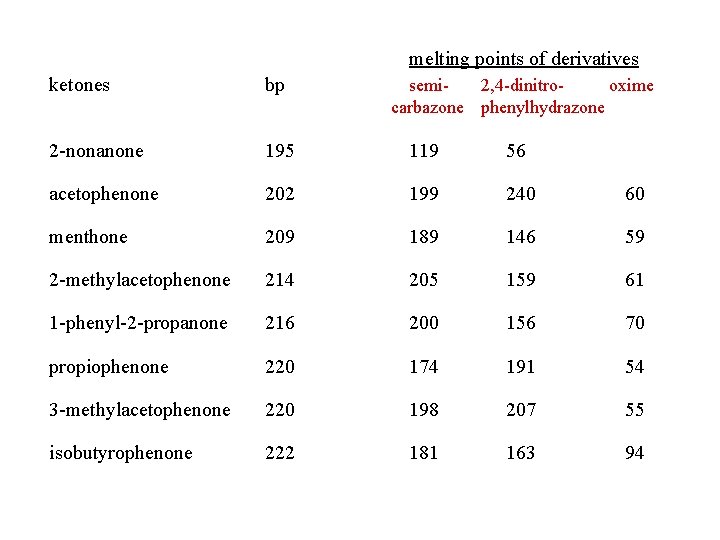
melting points of derivatives ketones bp 2 -nonanone 195 119 56 acetophenone 202 199 240 60 menthone 209 189 146 59 2 -methylacetophenone 214 205 159 61 1 -phenyl-2 -propanone 216 200 156 70 propiophenone 220 174 191 54 3 -methylacetophenone 220 198 207 55 isobutyrophenone 222 181 163 94 semi 2, 4 -dinitrooxime carbazone phenylhydrazone

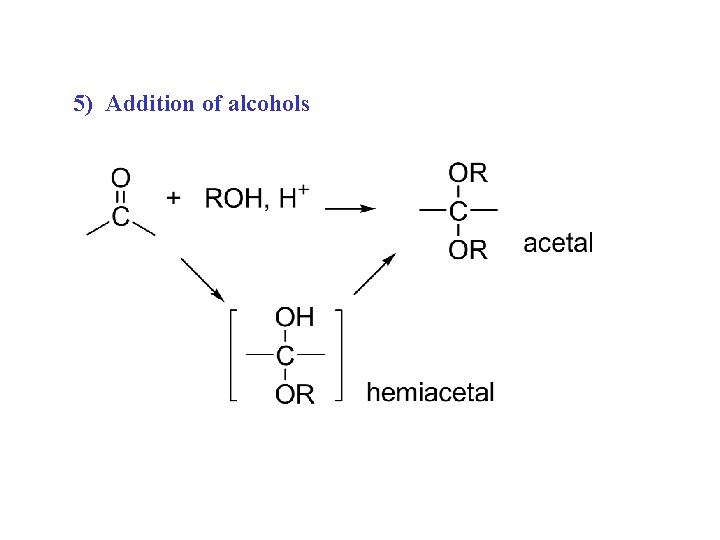
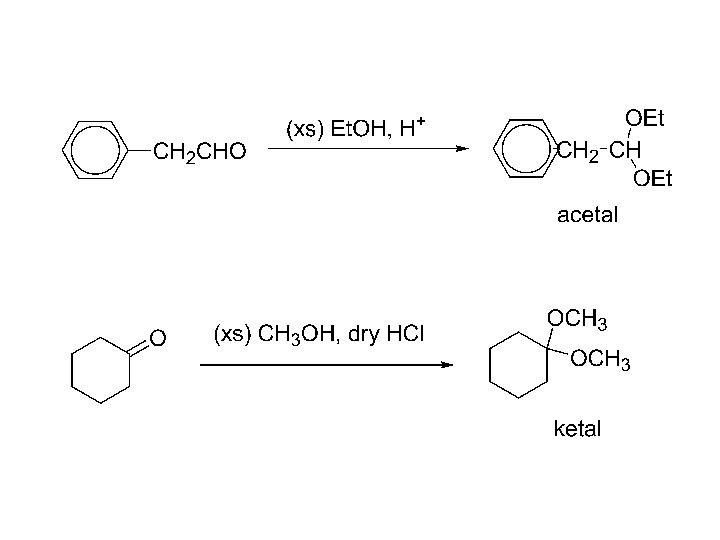
5) Addition of alcohols




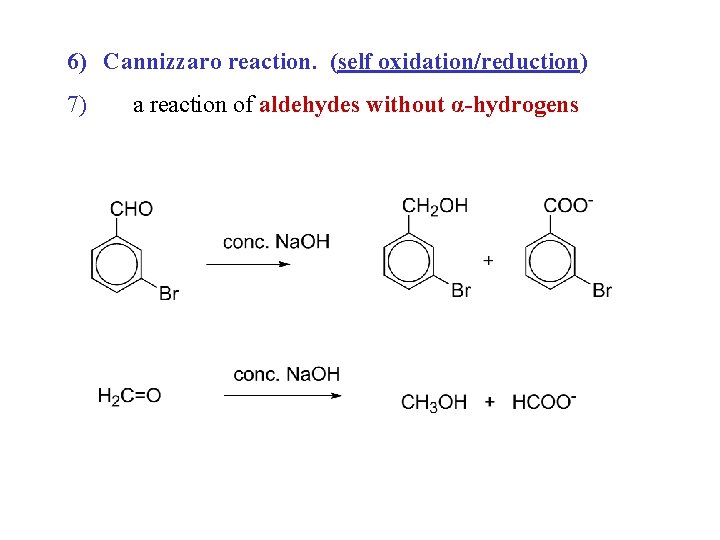
6) Cannizzaro reaction. (self oxidation/reduction) 7) a reaction of aldehydes without α-hydrogens

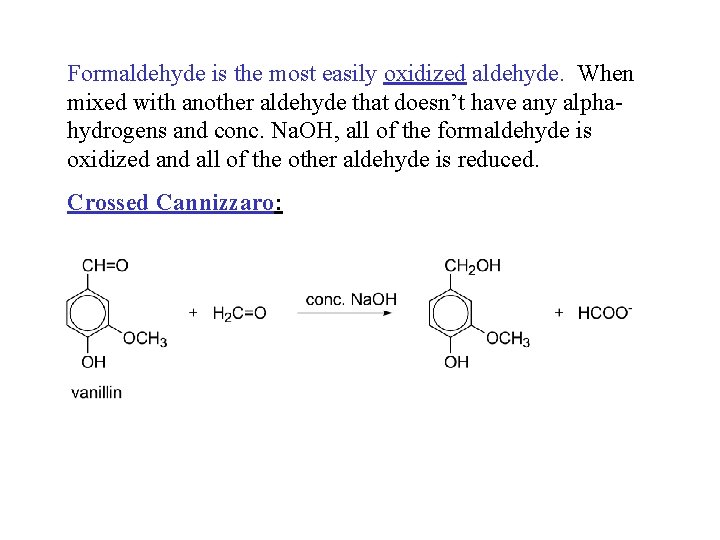
Formaldehyde is the most easily oxidized aldehyde. When mixed with another aldehyde that doesn’t have any alphahydrogens and conc. Na. OH, all of the formaldehyde is oxidized and all of the other aldehyde is reduced. Crossed Cannizzaro:

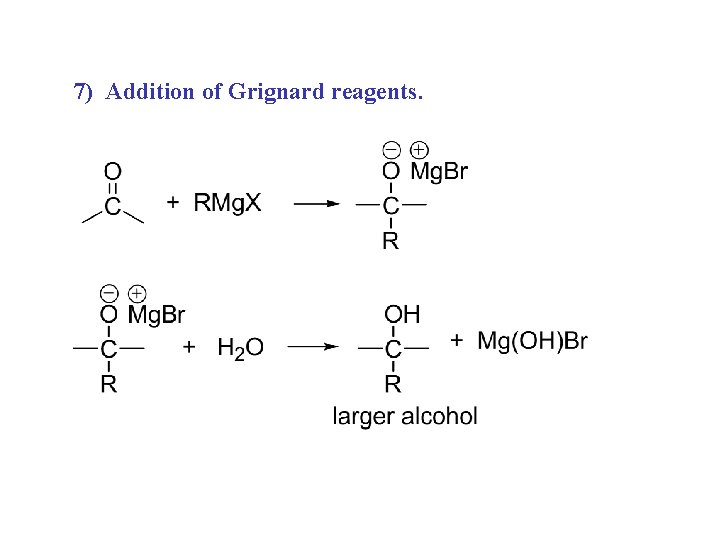
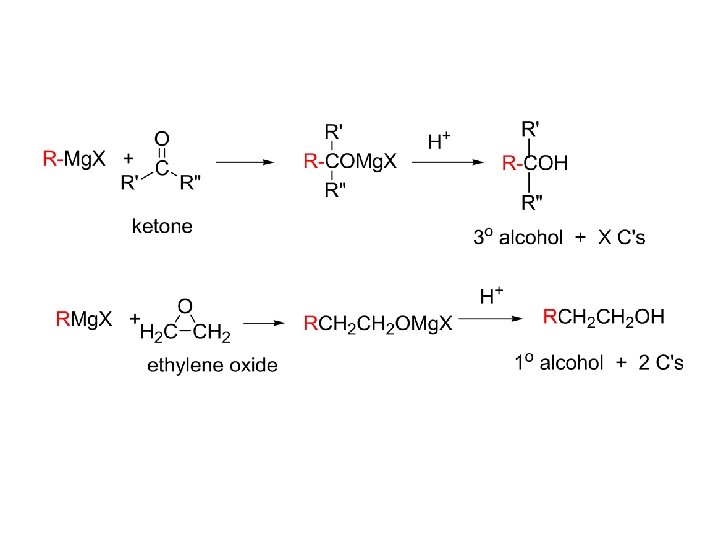
7) Addition of Grignard reagents.

1) 2)

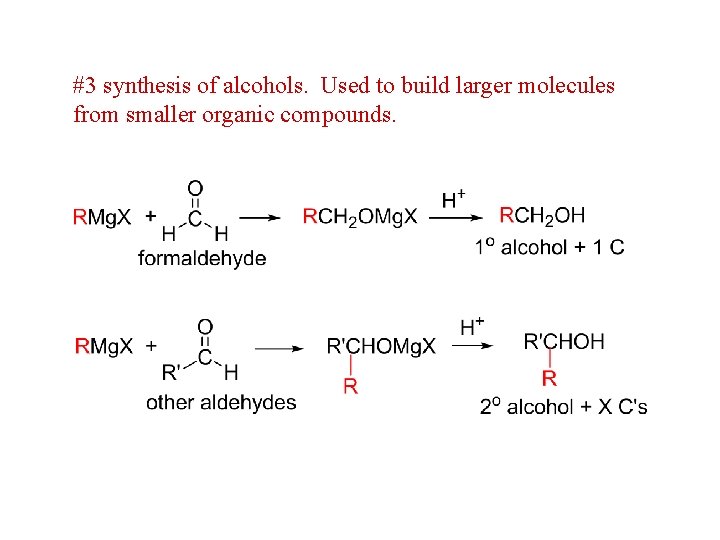
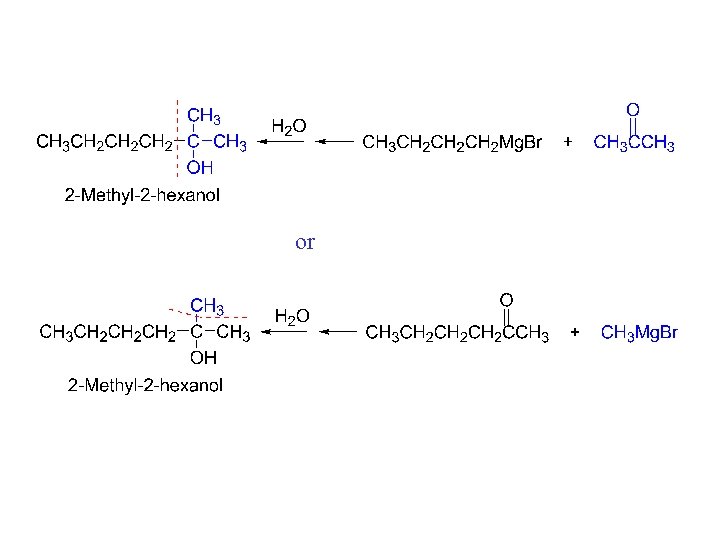
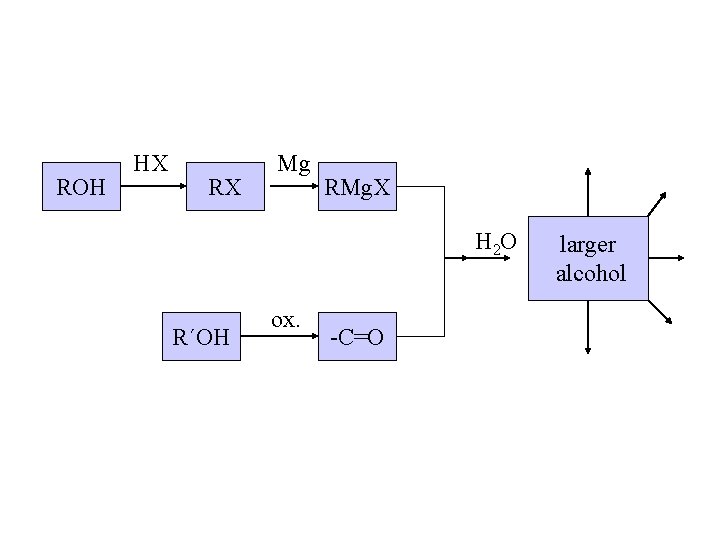
#3 synthesis of alcohols. Used to build larger molecules from smaller organic compounds.


Aldehydes & ketones, reactions: 1) Oxidation 2) Reduction 3) Addition of cyanide 4) Addition of derivatives of ammonia 5) Addition of alcohols 6) Cannizzaro reaction 7) Addition of Grignard reagents 8) 8) (Alpha-halogenation of ketones) 9) 9) (Addition of carbanions)

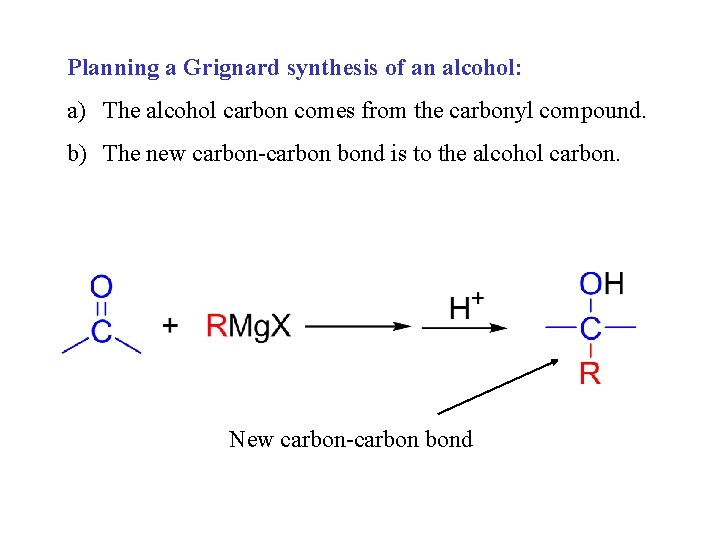
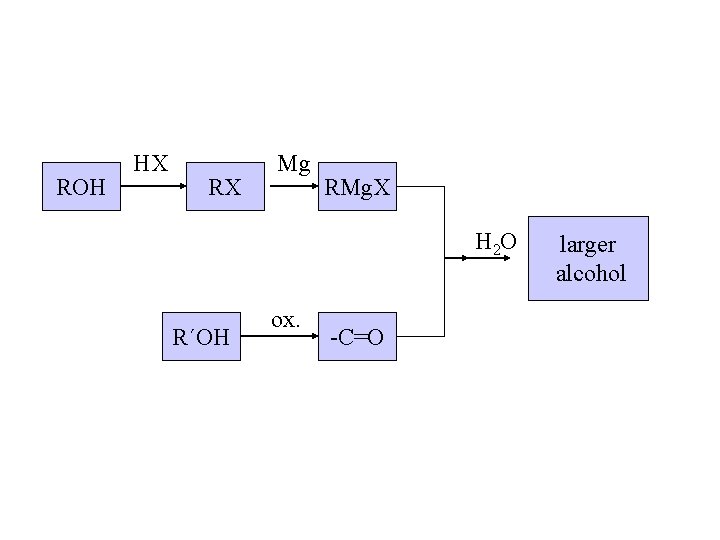
Planning a Grignard synthesis of an alcohol: a) The alcohol carbon comes from the carbonyl compound. b) The new carbon-carbon bond is to the alcohol carbon. New carbon-carbon bond

“The Grignard Song” (sung to the tune of “America the Beautiful”) Harry Wasserman The carbonyl is polarized, the carbon end is plus. A nucleophile will thus attack the carbon nucleus. The Grignard yields an alcohol of types there are but three. It makes a bond that corresponds from “C” to shining “C. ”

or

ROH HX RX Mg RMg. X H 2 O R´OH ox. -C=O larger alcohol

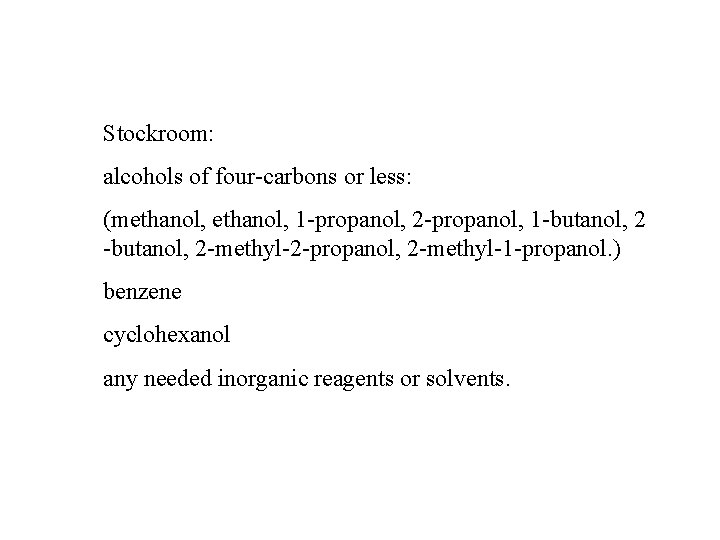
Stockroom: alcohols of four-carbons or less: (methanol, 1 -propanol, 2 -propanol, 1 -butanol, 2 -methyl-2 -propanol, 2 -methyl-1 -propanol. ) benzene cyclohexanol any needed inorganic reagents or solvents.

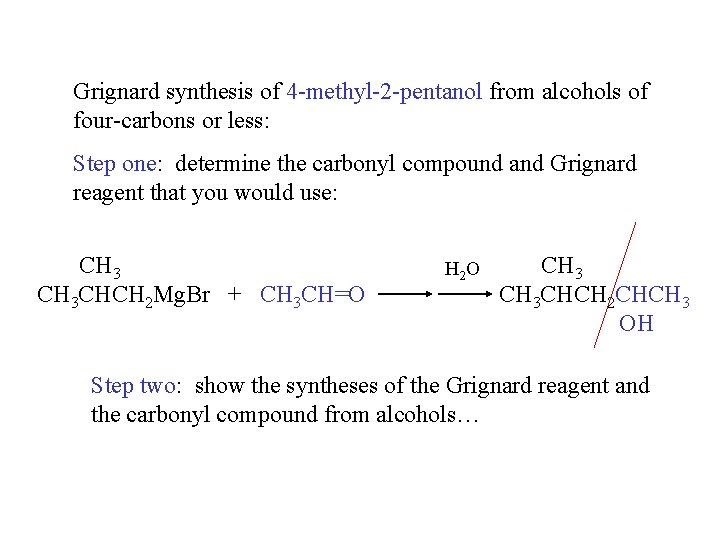
Grignard synthesis of 4 -methyl-2 -pentanol from alcohols of four-carbons or less: Step one: determine the carbonyl compound and Grignard reagent that you would use: CH 3 CHCH 2 Mg. Br + CH 3 CH=O H 2 O CH 3 CHCH 2 CHCH 3 OH Step two: show the syntheses of the Grignard reagent and the carbonyl compound from alcohols…

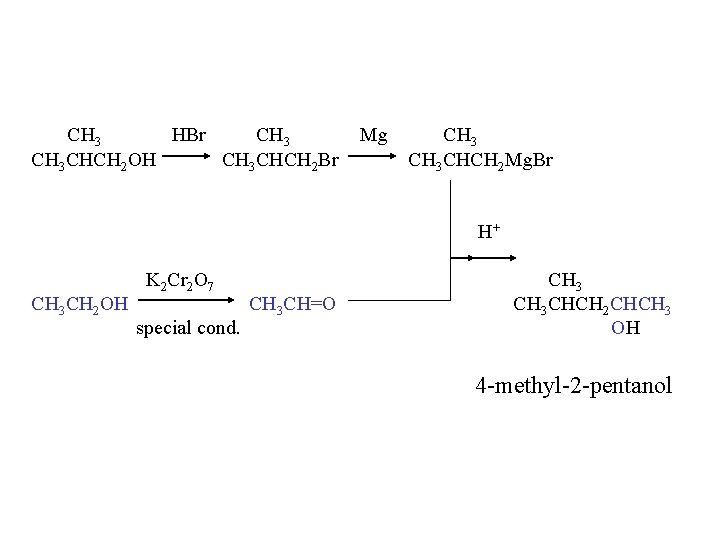
CH 3 HBr CH 3 CHCH 2 OH CH 3 CHCH 2 Br Mg CH 3 CHCH 2 Mg. Br H+ CH 3 CH 2 OH K 2 Cr 2 O 7 special cond. CH 3 CH=O CH 3 CHCH 2 CHCH 3 OH 4 -methyl-2 -pentanol

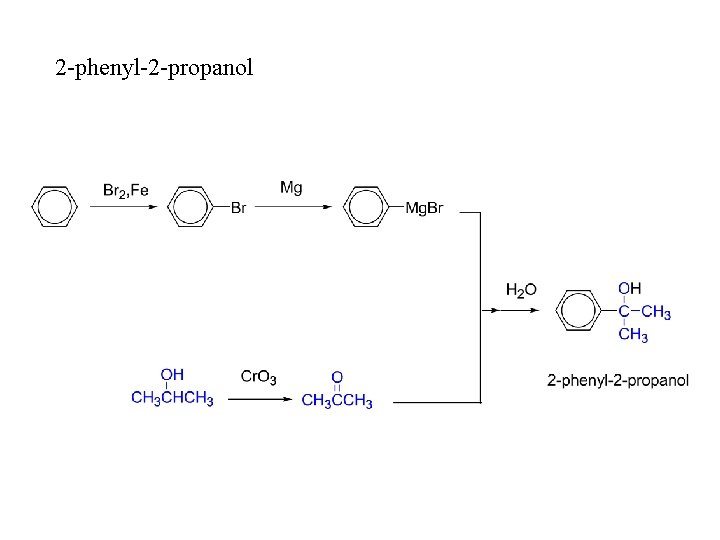
2 -phenyl-2 -propanol

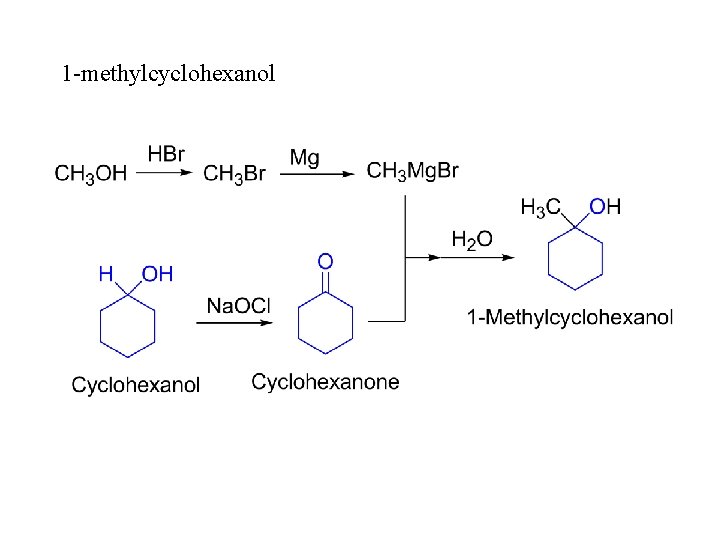
1 -methylcyclohexanol

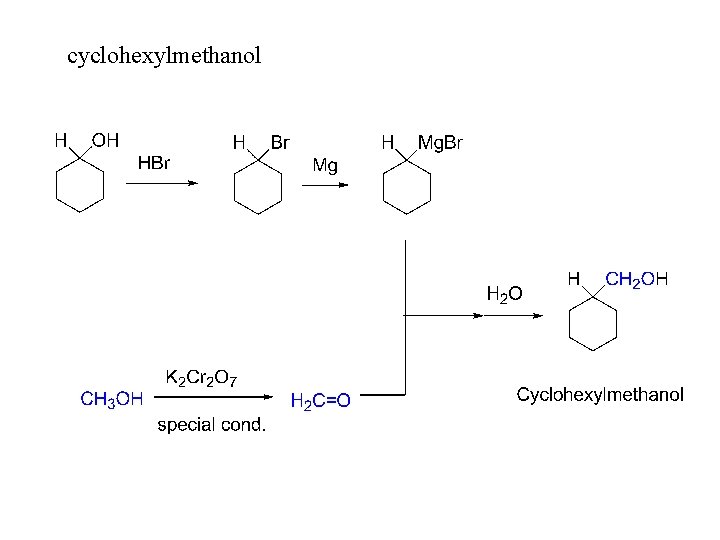
cyclohexylmethanol

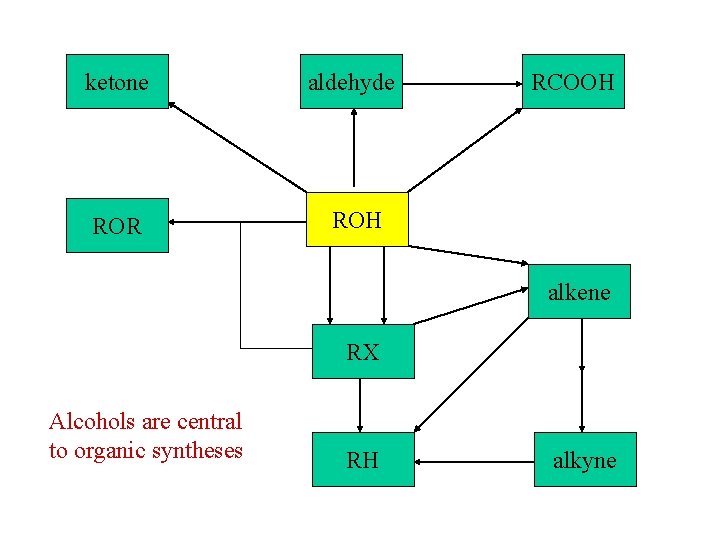
ketone aldehyde ROR ROH RCOOH alkene RX Alcohols are central to organic syntheses RH alkyne

ROH HX RX Mg RMg. X H 2 O R´OH ox. -C=O larger alcohol

Using the Grignard synthesis of alcohols we can make any alcohol that we need from a few simple alcohols. From those alcohols we can synthesize alkanes, alkenes, alkyl halides, ethers, aldehydes, ketones, carboxylic acids… eg. Outline all steps in a possible laboratory synthesis of 3 -methyl-1 -butene from alcohols of four carbons or less. CH 3 CHCH=CH 2

Retrosynthesis: alkenes, syntheses: 1. Dehydrohalogenation of an alkyl halide 2. Dehydration of an alcohol 3. Dehalogenation of a vicinal dihalide 4. Reduction of an alkyne Methods 3 & 4 start with compounds that are in turn made from alkenes.

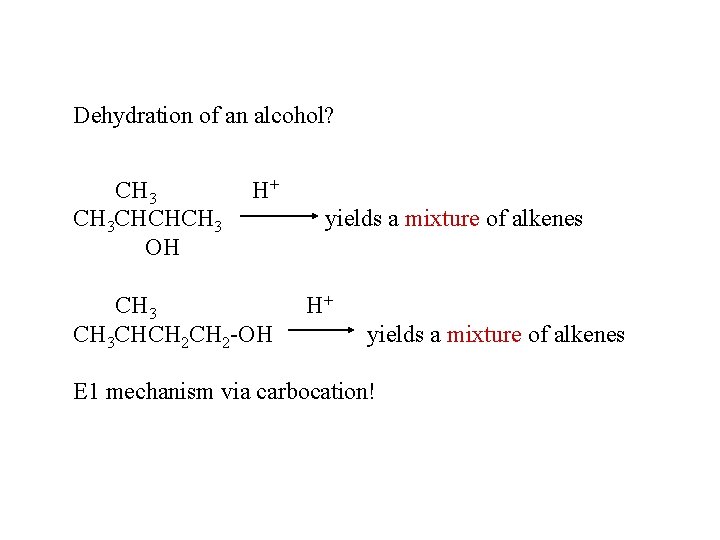
Dehydration of an alcohol? CH 3 CHCHCH 3 OH H+ CH 3 CHCH 2 -OH yields a mixture of alkenes H+ yields a mixture of alkenes E 1 mechanism via carbocation!

Dehydrohalogenation of an alkyl halide? CH 3 CHCHCH 3 Br KOH(alc) CH 3 CHCH 2 -Br yields a mixture of alkenes KOH(alc) CH 3 CHCH=CH 2 only product E 2 mechanism, no carbocation, no rearrangement

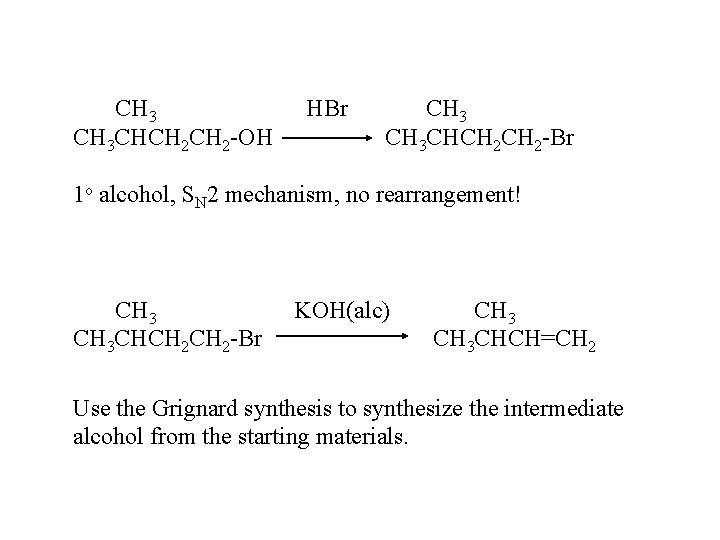
CH 3 CHCH 2 -OH HBr CH 3 CHCH 2 -Br 1 o alcohol, SN 2 mechanism, no rearrangement! CH 3 CHCH 2 -Br KOH(alc) CH 3 CHCH=CH 2 Use the Grignard synthesis to synthesize the intermediate alcohol from the starting materials.

CH 3 CHCH 2 -OH CH 3 OH PBr 3 K 2 Cr 2 O 7 special cond. CH 3 CHCH 2 Br Mg H 2 C=O CH 3 CHCH 2 Mg. Br H 2 O CH 3 CHCH 2 -OH HBr CH 3 CHCH=CH 2 KOH(alco) CH 3 CHCH 2 -Br