Reactions of Acids Neutralisation The reaction between an

Reactions of Acids

Neutralisation • The reaction between an acid and a base is called a neutralisation. • A salt and water are formed. • The general reaction is: acid + base → salt + Water

Examples of Neutralisation • Hydrochloric acid and sodium hydroxide Hydrochloric acid + Sodium Hydroxide → Sodium chloride + Water HCl + Na. OH → Na. Cl + H 2 O

Examples of Neutralisation • Sulfuric acid and ammonium hydroxide Sulfuric acid + Ammonium Hydroxide → Ammonium Sulfate + Water H 2 SO 4 + NH 4 OH → (NH 4) 2 SO 4 + H 2 O

Examples of Neutralisation • Nitric acid and ammonium hydroxide Nitric acid + Ammonium Hydroxide → Ammonium Nitrate + Water HNO 3 + NH 4 OH → NH 4 NO 3 + H 2 O

Acids and Metals • Acids such as sulfuric acid and hydrochloric acid react with many metals. • Hydrogen gas is given off. • The general reaction is: acid + metal → salt + Hydrogen
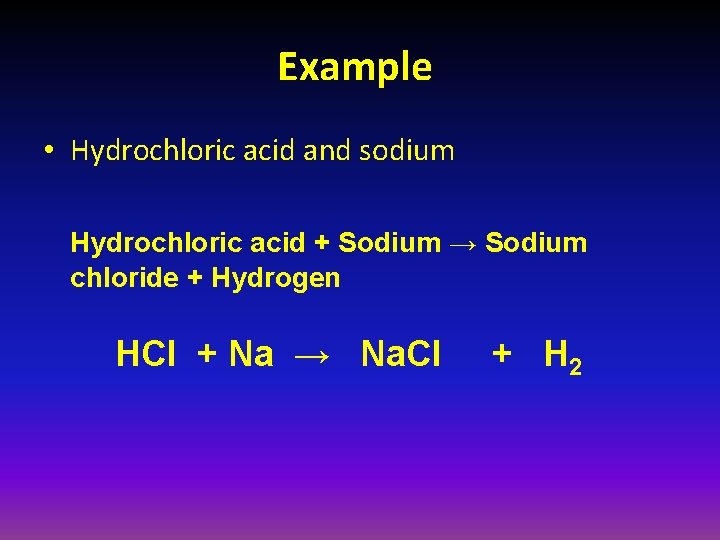
Example • Hydrochloric acid and sodium Hydrochloric acid + Sodium → Sodium chloride + Hydrogen HCl + Na → Na. Cl + H 2

Example • Sulfuric acid and Magnesium Sulfuric acid + Magnesium → Magnesium Sulfate + Hydrogen H 2 SO 4 + Mg → Mg. SO 4 + H 2

Examples • Nitric acid and Zinc Nitric acid + Zinc → Zinc Nitrate + Hydrogen HNO 3 + NH 4 OH → Zn(NO 3 )2 + H 2

Acids and Metal Oxides • Acids react with metal oxides to form a salt and water • acid + metal oxide → salt + water

Example Hydrochloric acid + Calcium Oxide → Calcium chloride + Water HCl + Ca. O → Ca. Cl 2 + H 2 O

Acids and Metal Hydroxides • Acids react with metal hydroxides to form a salt and water • acid + metal hydroxide → salt + water
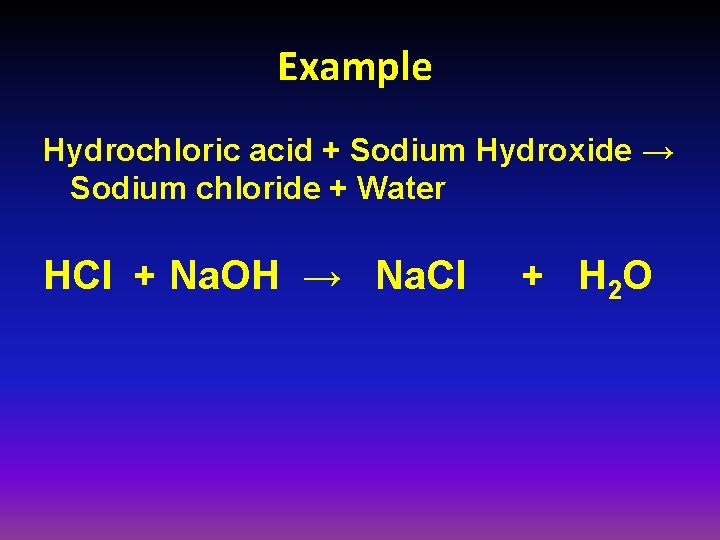
Example Hydrochloric acid + Sodium Hydroxide → Sodium chloride + Water HCl + Na. OH → Na. Cl + H 2 O

Acids and Carbonates • Acids react with carbonates to produce a salt, carbon dioxide gas and water. • The general reaction is: acid + Carbonate → salt + carbon dioxide + water
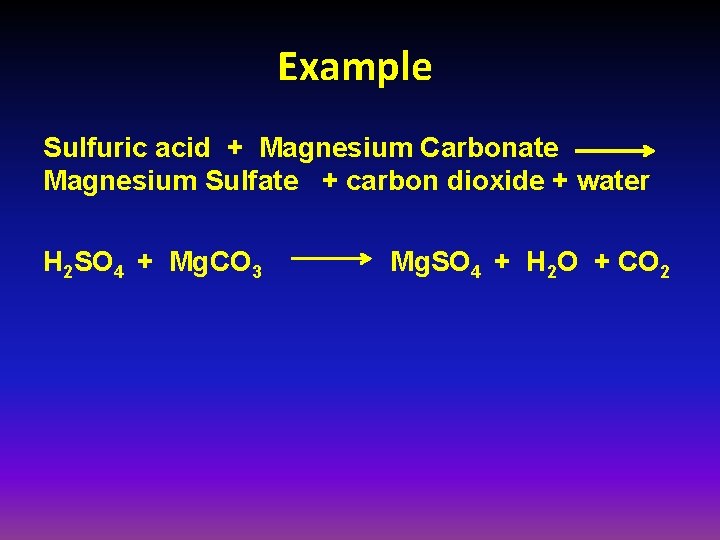
Example Sulfuric acid + Magnesium Carbonate Magnesium Sulfate + carbon dioxide + water H 2 SO 4 + Mg. CO 3 Mg. SO 4 + H 2 O + CO 2
- Slides: 15