Reactions Metals and acids The diagram below shows
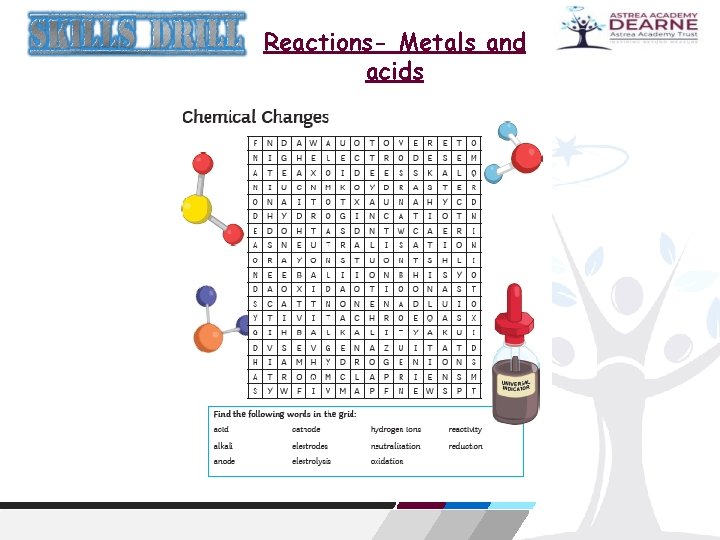
Reactions- Metals and acids
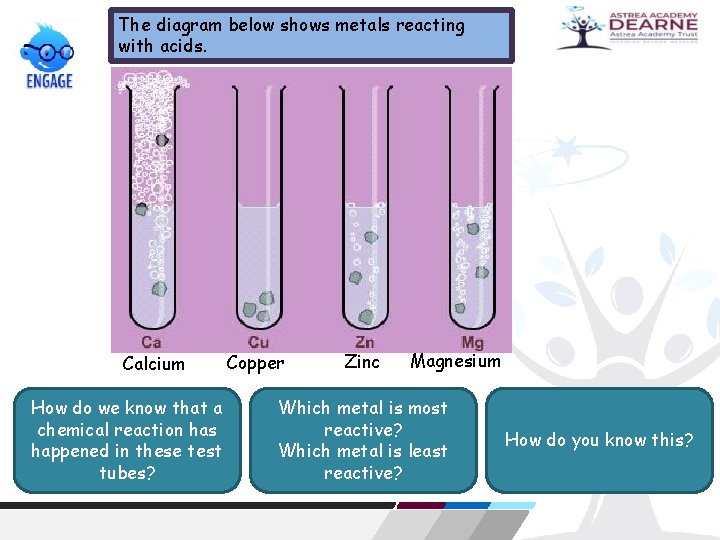
The diagram below shows metals reacting with acids. Calcium How do we know that a chemical reaction has happened in these test tubes? Copper Zinc Magnesium Which metal is most reactive? Which metal is least reactive? How do you know this?

Chemical reactions Atoms are rearranged in a chemical reaction. The substances that: react together are called the reactants are formed in the reaction are called the products No atoms are created or destroyed in a chemical reaction. This means that the total mass of the reactants is the same as the total mass of the products. We say that mass is conserved in a chemical reaction.
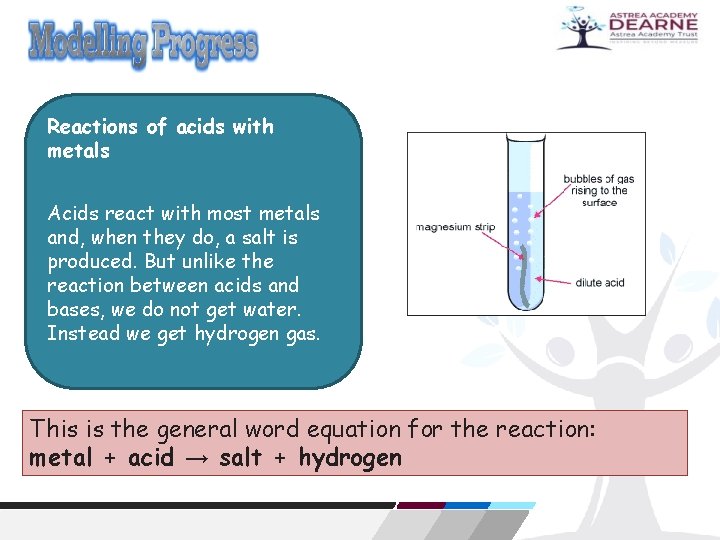
Reactions of acids with metals Acids react with most metals and, when they do, a salt is produced. But unlike the reaction between acids and bases, we do not get water. Instead we get hydrogen gas. This is the general word equation for the reaction: metal + acid → salt + hydrogen
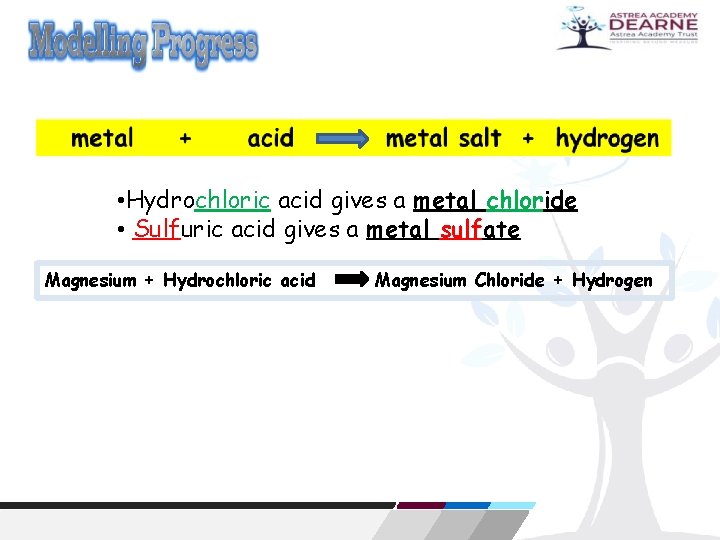
• Hydrochloric acid gives a metal chloride • Sulfuric acid gives a metal sulfate Magnesium + Hydrochloric acid Magnesium Chloride + Hydrogen

What are they testing for? What did they use? What was the result?
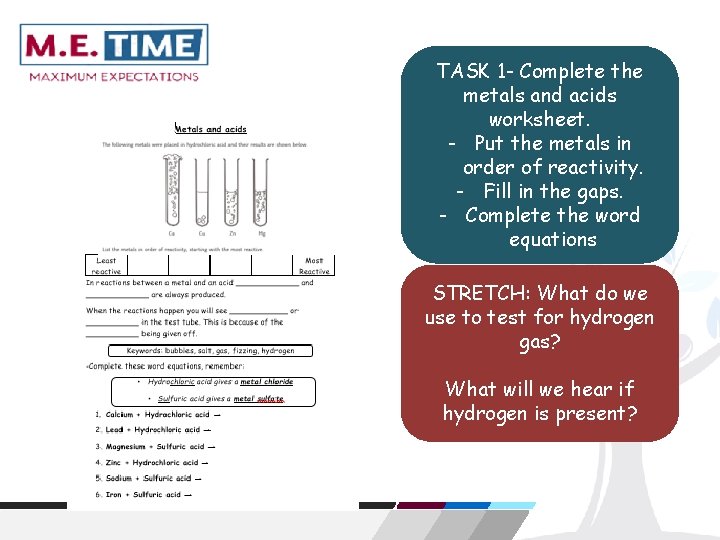
TASK 1 - Complete the metals and acids worksheet. - Put the metals in order of reactivity. - Fill in the gaps. - Complete the word equations STRETCH: What do we use to test for hydrogen gas? What will we hear if hydrogen is present?

1. Calcium + Hydrochloric acid → Calcium Chloride + Hydrogen 2. Lead + Hydrochloric acid →Lead Chloride + Hydrogen 3. Magnesium + Sulfuric acid →Magnesium sulfate + Hydrogen 4. Zinc + Hydrochloric acid → Zinc Chloride + Hydrogen 5. Sodium + Sulfuric acid → Sodium Sulfate+ Hydrogen 6. Iron + Sulfuric acid → Iron Sulfate + Hydrogen
The three main greenhouse gases are: C____ d____ M__t__ne Sulfu_ d______ The greenhouse effect causes… When we burn fossil fuels pollutants are released into the air. These are: When a metal reacts with an acid what gets produced?
- Slides: 10