Reactions in Aqueous Solutions Chapter 4 GENERAL PROPERTIES
























- Slides: 61

Reactions in Aqueous Solutions Chapter 4

GENERAL PROPERTIES

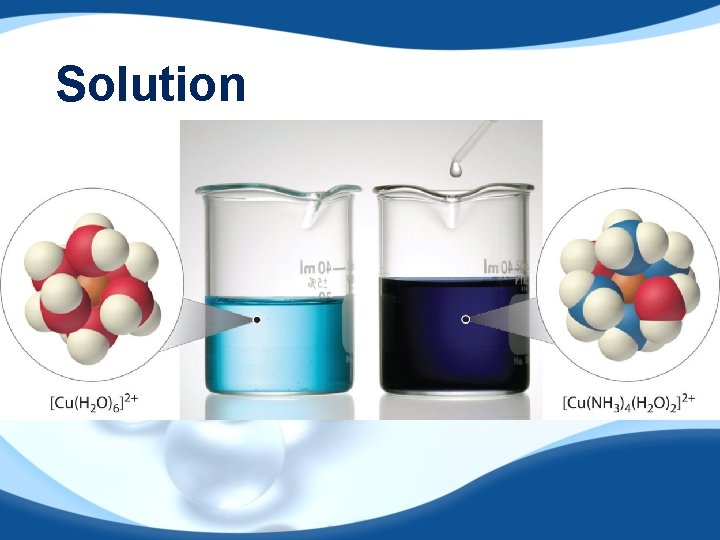
Solution

A solution is a homogenous mixture of 2 or more substances. The solute is (are) the substance(s) present in the smaller amount(s). The solvent is the substance present in the larger amount. Solution Solvent Solute Soft drink (l) H 2 O Sugar, CO 2 Air (g) N 2 O 2, Ar, CH 4 Soft solder (s) Pb Sn 4 aqueous solutions of KMn. O 4

Conduct electricity in solution? Cations (+) and Anions (-) ELECTROLYTE

An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity.

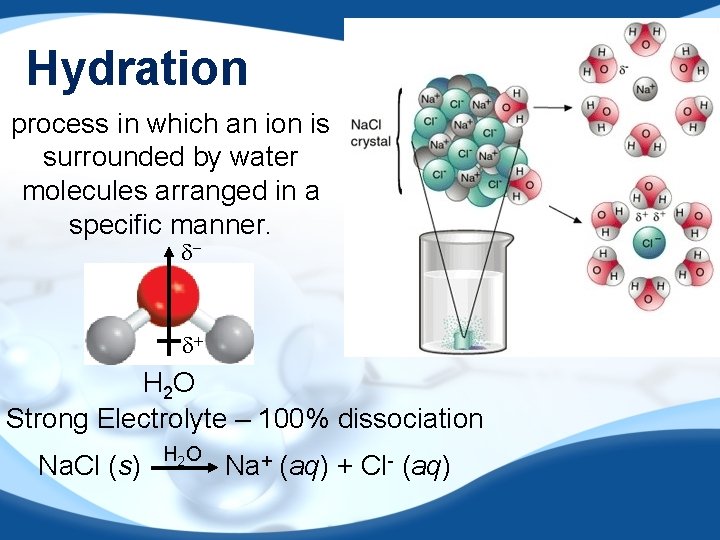
Hydration process in which an ion is surrounded by water molecules arranged in a specific manner. d- d+ H 2 O Strong Electrolyte – 100% dissociation Na. Cl (s) H 2 O Na+ (aq) + Cl- (aq)

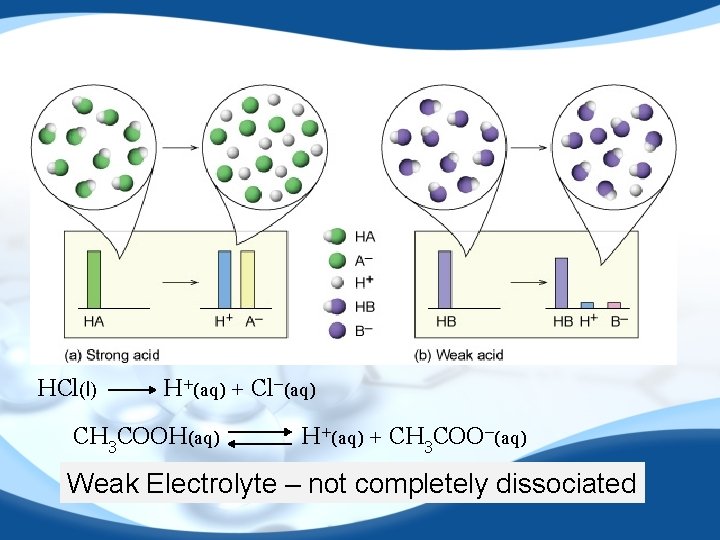
HCl(l) H+(aq) + Cl−(aq) CH 3 COOH(aq) H+(aq) + CH 3 COO−(aq) Weak Electrolyte – not completely dissociated

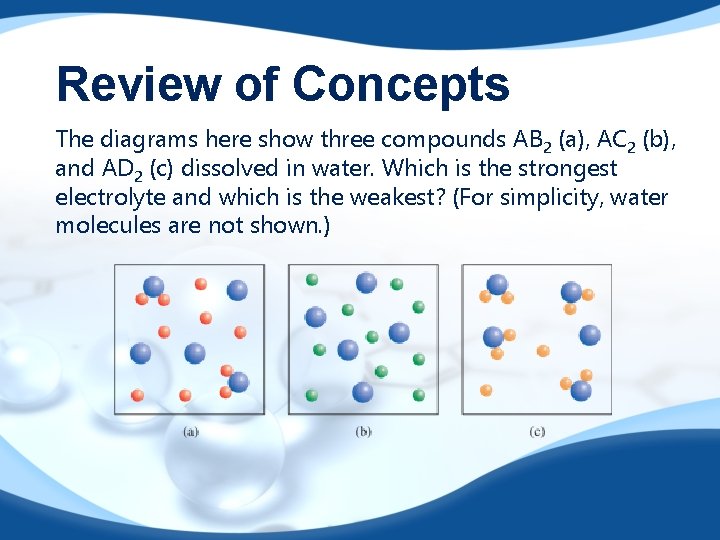
Review of Concepts The diagrams here show three compounds AB 2 (a), AC 2 (b), and AD 2 (c) dissolved in water. Which is the strongest electrolyte and which is the weakest? (For simplicity, water molecules are not shown. )

PRECIPITATE REACTIONS

Precipitate – insoluble solid that separates from solution Cd. S Pb. S Ni(OH)2 Al(OH)3

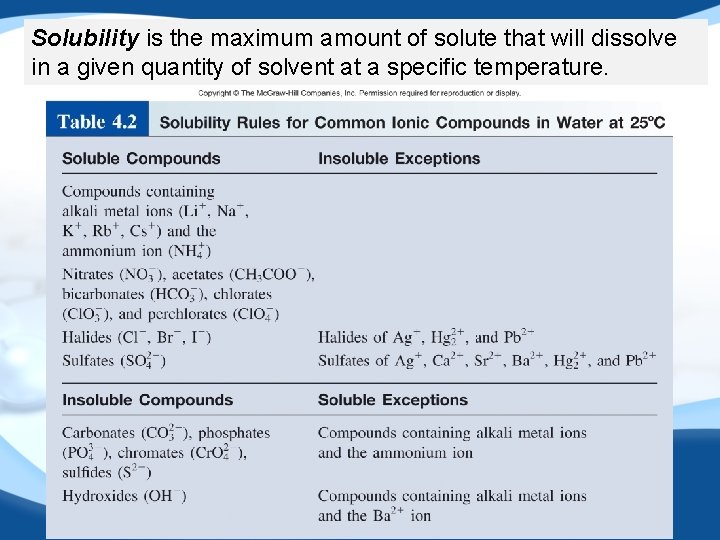
Solubility is the maximum amount of solute that will dissolve in a given quantity of solvent at a specific temperature.

Example: 4. 1 Classify the following ionic compounds as soluble or insoluble: (a) silver sulfate (Ag 2 SO 4) (b) calcium carbonate (Ca. CO 3) (c) sodium phosphate (Na 3 PO 4).


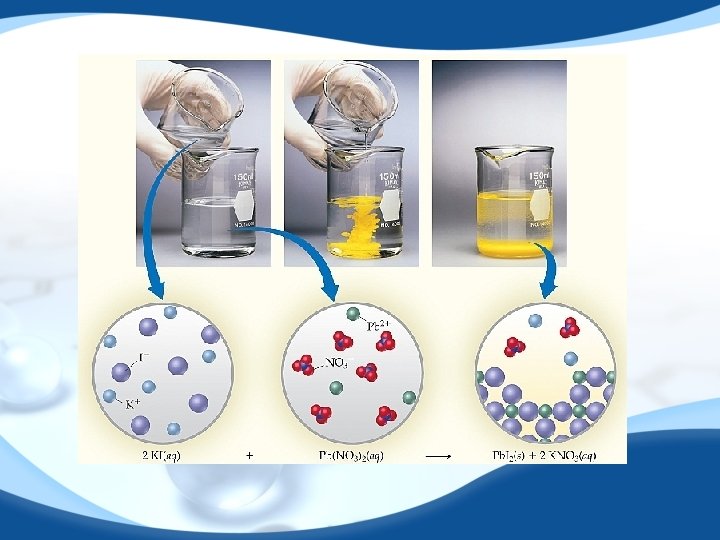
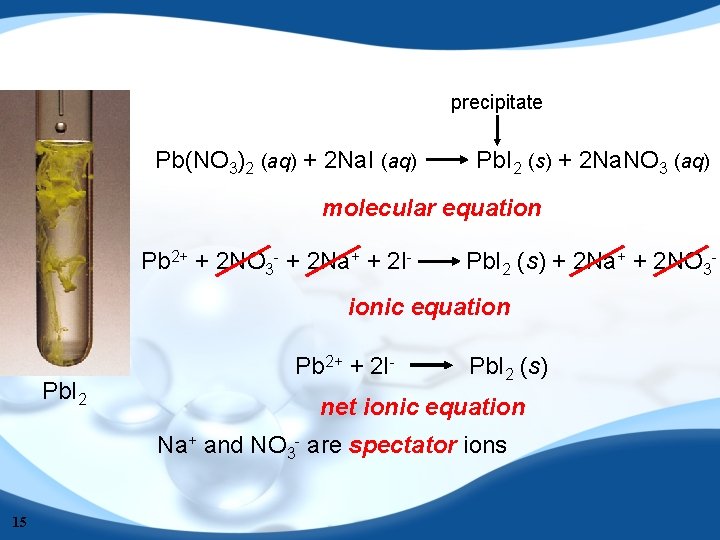
precipitate Pb(NO 3)2 (aq) + 2 Na. I (aq) Pb. I 2 (s) + 2 Na. NO 3 (aq) molecular equation Pb 2+ + 2 NO 3 - + 2 Na+ + 2 I- Pb. I 2 (s) + 2 Na+ + 2 NO 3 - ionic equation Pb. I 2 Pb 2+ + 2 I- Pb. I 2 (s) net ionic equation Na+ and NO 3 - are spectator ions 15

Writing Net Ionic Equations 1. Write the balanced molecular equation. 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation. 4. Check that charges and number of atoms are balanced in the net ionic equation. 16

Example: 4. 2 Predict what happens when a potassium phosphate (K 3 PO 4) solution is mixed with a calcium nitrate [Ca(NO 3)2] solution. Write a net ionic equation for the reaction.

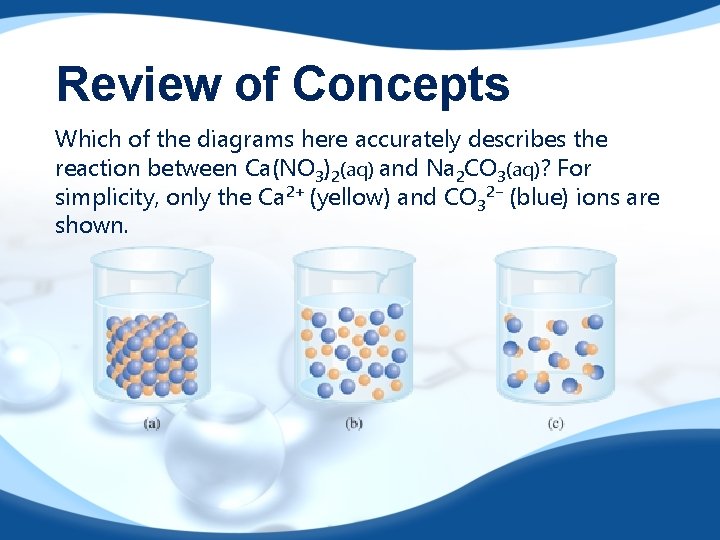
Review of Concepts Which of the diagrams here accurately describes the reaction between Ca(NO 3)2(aq) and Na 2 CO 3(aq)? For simplicity, only the Ca 2+ (yellow) and CO 32− (blue) ions are shown.

ACID-BASE REACTIONS

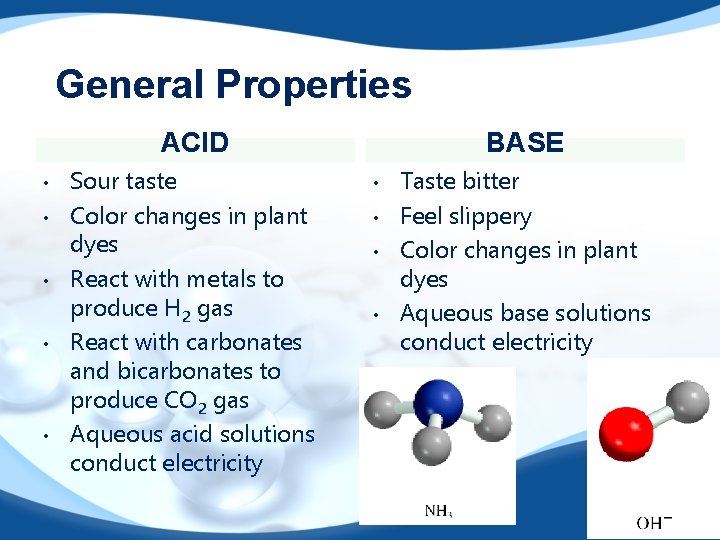
General Properties ACID • • • Sour taste Color changes in plant dyes React with metals to produce H 2 gas React with carbonates and bicarbonates to produce CO 2 gas Aqueous acid solutions conduct electricity BASE • • Taste bitter Feel slippery Color changes in plant dyes Aqueous base solutions conduct electricity

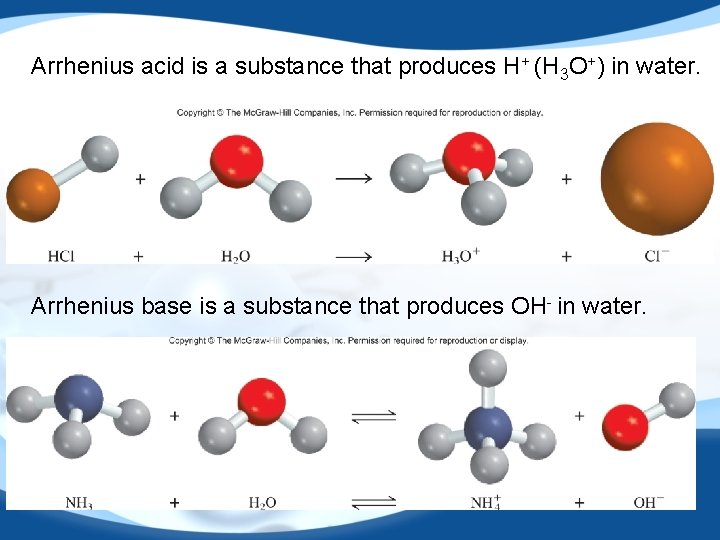
Arrhenius acid is a substance that produces H+ (H 3 O+) in water. Arrhenius base is a substance that produces OH- in water. 21

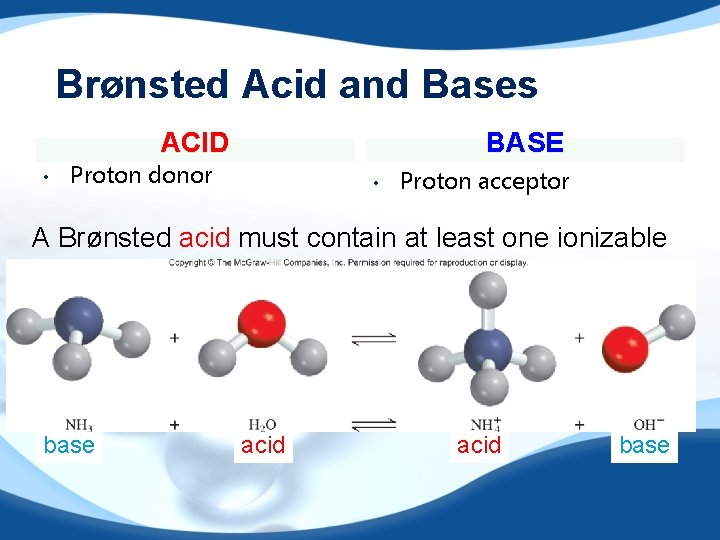
Brønsted Acid and Bases ACID • BASE Proton donor • Proton acceptor A Brønsted acid must contain at least one ionizable proton! base acid base

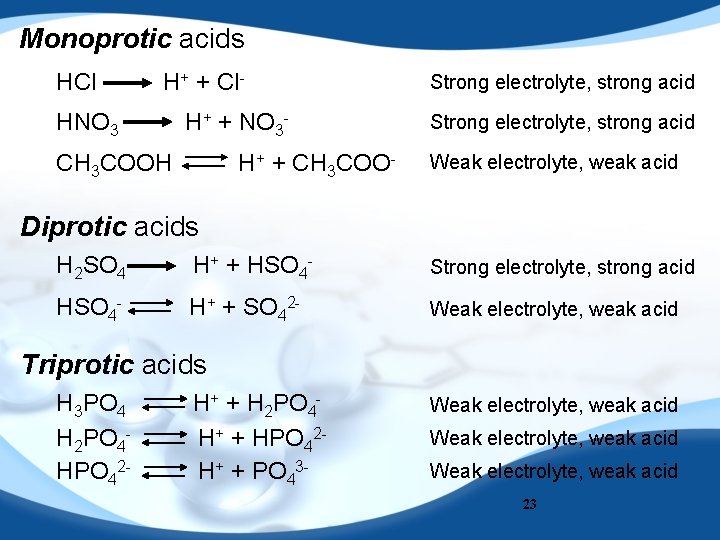
Monoprotic acids HCl H+ + Cl- HNO 3 H+ + NO 3 - CH 3 COOH H+ + CH 3 COO- Strong electrolyte, strong acid Weak electrolyte, weak acid Diprotic acids H 2 SO 4 H+ + HSO 4 - Strong electrolyte, strong acid HSO 4 - H+ + SO 42 - Weak electrolyte, weak acid Triprotic acids H 3 PO 4 H 2 PO 4 HPO 42 - H+ + H 2 PO 4 H+ + HPO 42 H+ + PO 43 - Weak electrolyte, weak acid 23

STRONG ACIDS HI HBr HCl. O 4 HCl H 2 SO 4 HNO 3 STRONG BASES Na. OH KOH Li. OH Rb. OH Cs. OH Ca(OH)2 Ba(OH)2 Sr(OH)2 Strong acids/bases are strong electrolytes and will completely dissociate in water.

Example: 4. 3 Classify each of the following species in aqueous solution as a Brønsted acid or base: (a) (b) (c) HBr

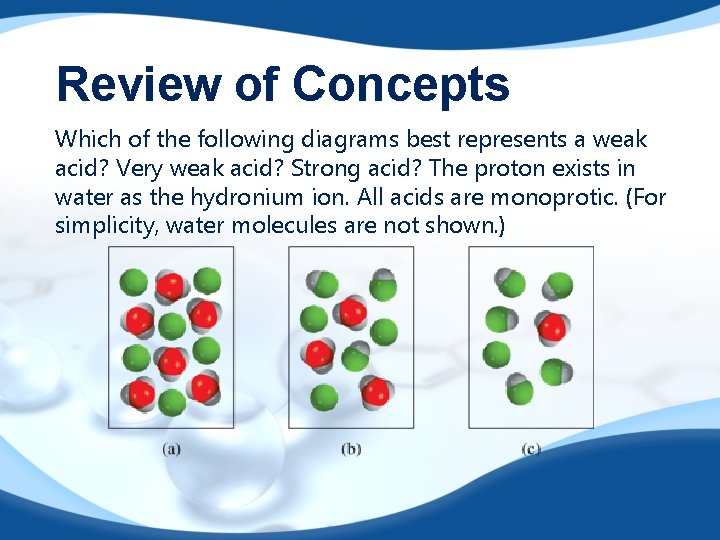
Review of Concepts Which of the following diagrams best represents a weak acid? Very weak acid? Strong acid? The proton exists in water as the hydronium ion. All acids are monoprotic. (For simplicity, water molecules are not shown. )

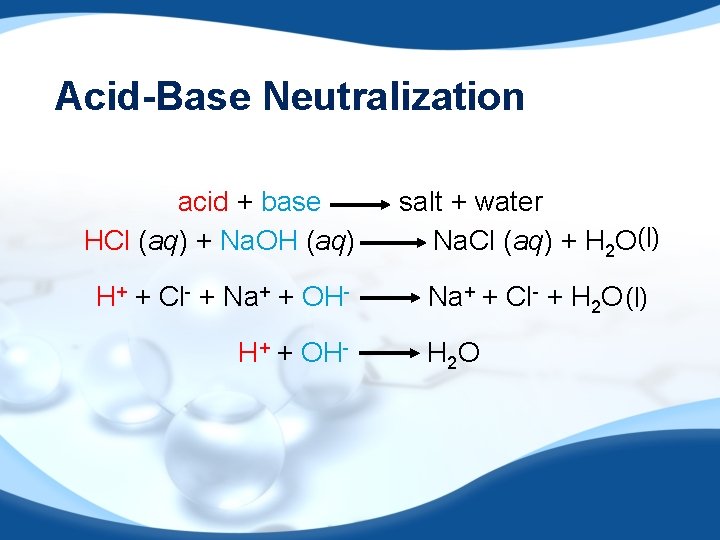
Acid-Base Neutralization acid + base HCl (aq) + Na. OH (aq) salt + water Na. Cl (aq) + H 2 O(l) H+ + Cl- + Na+ + OH- Na+ + Cl- + H 2 O (l) H+ + OH- H 2 O

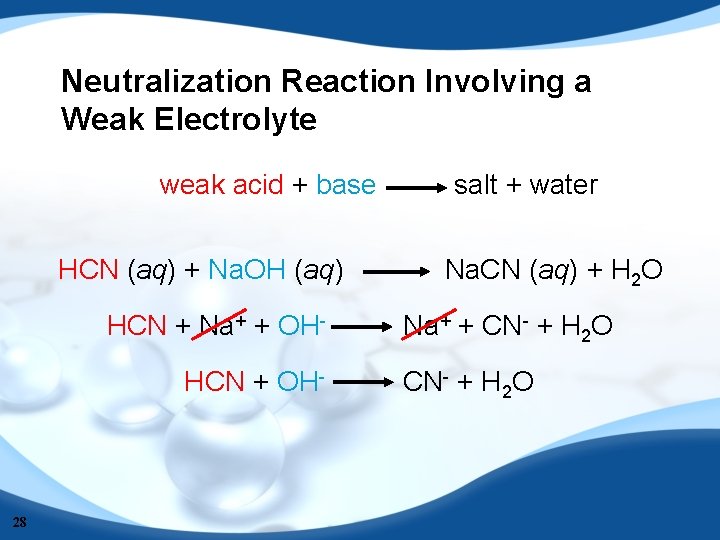
Neutralization Reaction Involving a Weak Electrolyte weak acid + base HCN (aq) + Na. OH (aq) HCN + Na+ + OHHCN + OH- 28 salt + water Na. CN (aq) + H 2 O Na+ + CN- + H 2 O

Example: 4. 4 Write molecular, ionic, and net ionic equations for each of the following acid-base reactions: (a) hydrobromic acid(aq) + barium hydroxide(aq) (b) sulfuric acid(aq) + potassium hydroxide(aq)

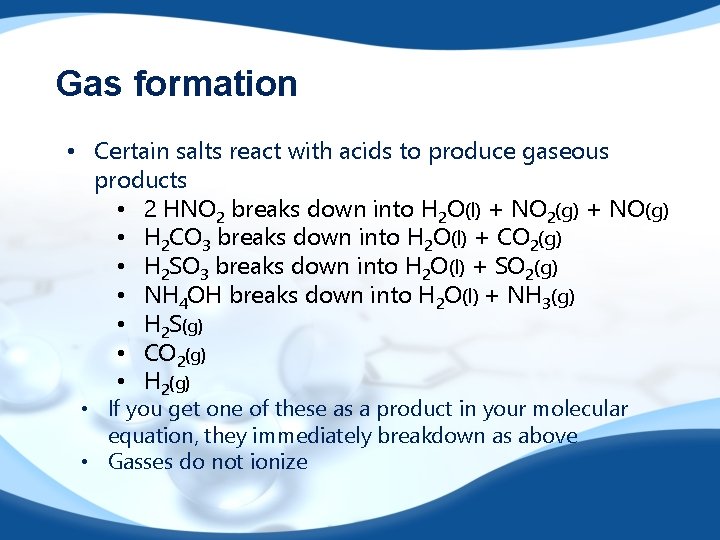
Gas formation • Certain salts react with acids to produce gaseous products • 2 HNO 2 breaks down into H 2 O(l) + NO 2(g) + NO(g) • H 2 CO 3 breaks down into H 2 O(l) + CO 2(g) • H 2 SO 3 breaks down into H 2 O(l) + SO 2(g) • NH 4 OH breaks down into H 2 O(l) + NH 3(g) • H 2 S(g) • CO 2(g) • H 2(g) • If you get one of these as a product in your molecular equation, they immediately breakdown as above • Gasses do not ionize

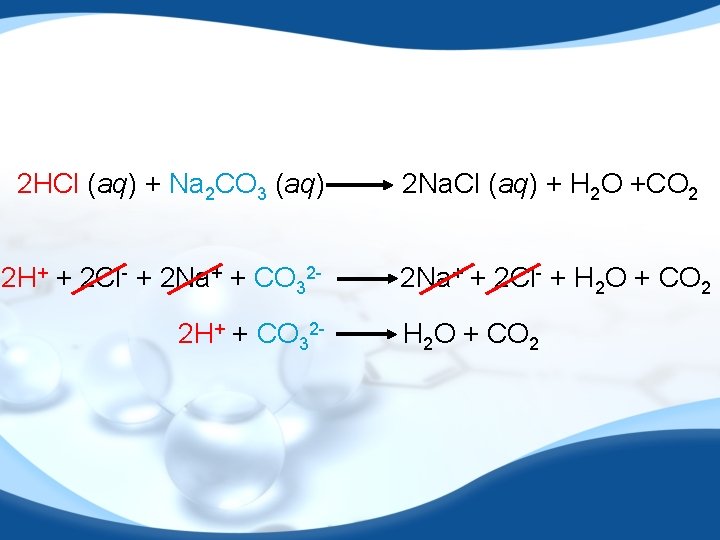
2 HCl (aq) + Na 2 CO 3 (aq) 2 H+ + 2 Cl- + 2 Na+ + CO 322 H+ + CO 32 - 2 Na. Cl (aq) + H 2 O +CO 2 2 Na+ + 2 Cl- + H 2 O + CO 2

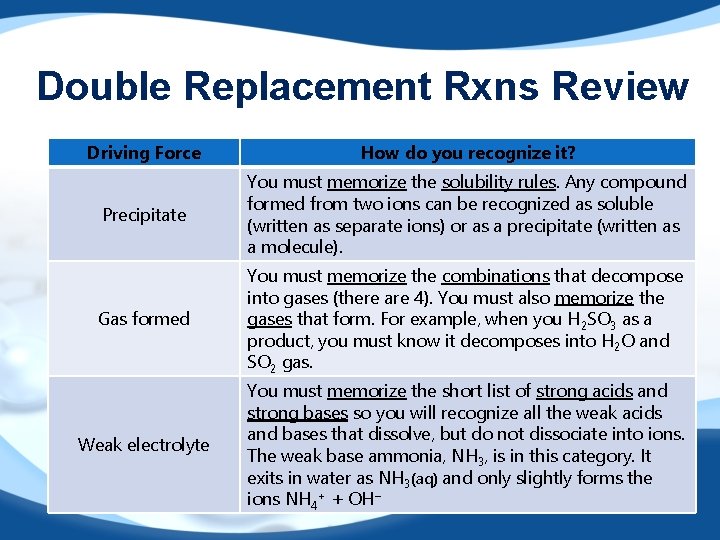
Double Replacement Rxns Review Driving Force How do you recognize it? Precipitate You must memorize the solubility rules. Any compound formed from two ions can be recognized as soluble (written as separate ions) or as a precipitate (written as a molecule). Gas formed You must memorize the combinations that decompose into gases (there are 4). You must also memorize the gases that form. For example, when you H 2 SO 3 as a product, you must know it decomposes into H 2 O and SO 2 gas. Weak electrolyte You must memorize the short list of strong acids and strong bases so you will recognize all the weak acids and bases that dissolve, but do not dissociate into ions. The weak base ammonia, NH 3, is in this category. It exits in water as NH 3(aq) and only slightly forms the ions NH 4+ + OH−

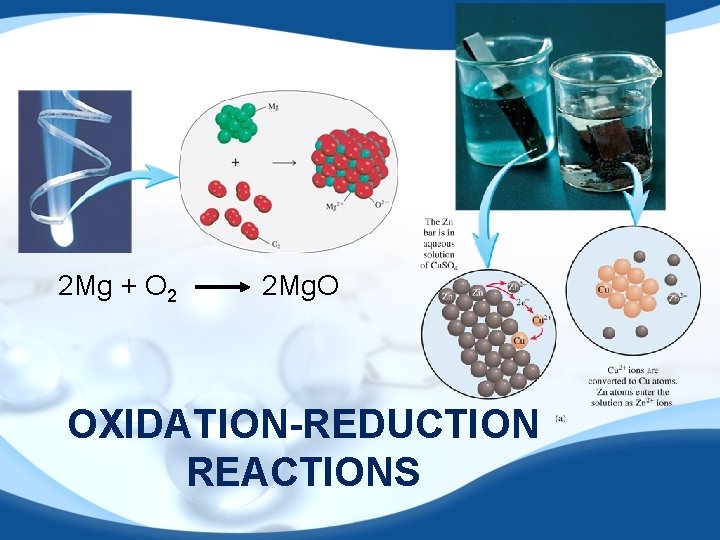
2 Mg + O 2 2 Mg. O OXIDATION-REDUCTION REACTIONS

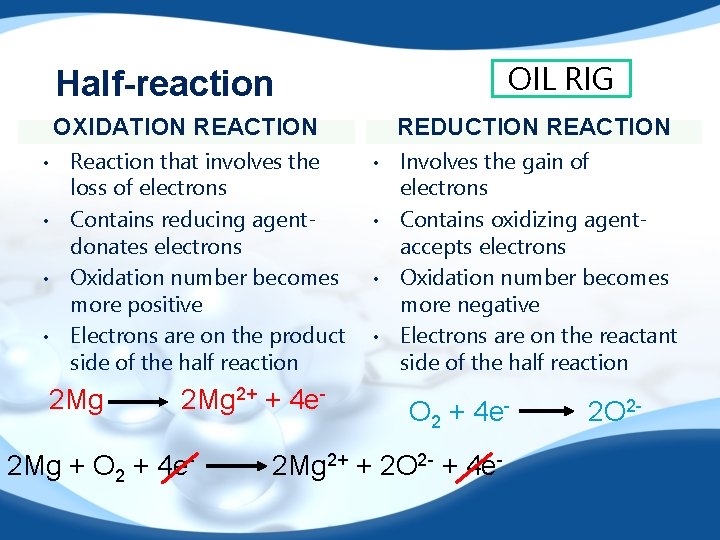
OIL RIG Half-reaction OXIDATION REACTION • • Reaction that involves the loss of electrons Contains reducing agentdonates electrons Oxidation number becomes more positive Electrons are on the product side of the half reaction 2 Mg 2+ + 4 e- 2 Mg + O 2 + 4 e- REDUCTION REACTION • • Involves the gain of electrons Contains oxidizing agentaccepts electrons Oxidation number becomes more negative Electrons are on the reactant side of the half reaction O 2 + 4 e- 2 Mg 2+ + 2 O 2 - + 4 e- 2 O 2 -

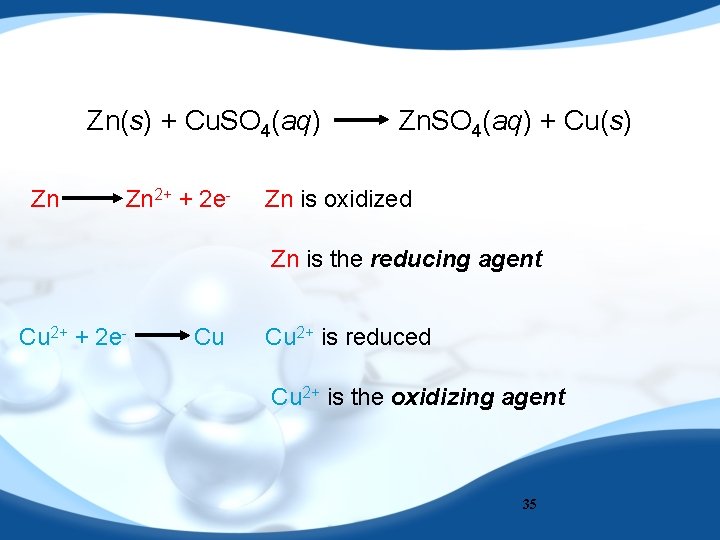
Zn(s) + Cu. SO 4(aq) Zn Zn 2+ + 2 e- Zn. SO 4(aq) + Cu(s) Zn is oxidized Zn is the reducing agent Cu 2+ + 2 e- Cu Cu 2+ is reduced Cu 2+ is the oxidizing agent 35

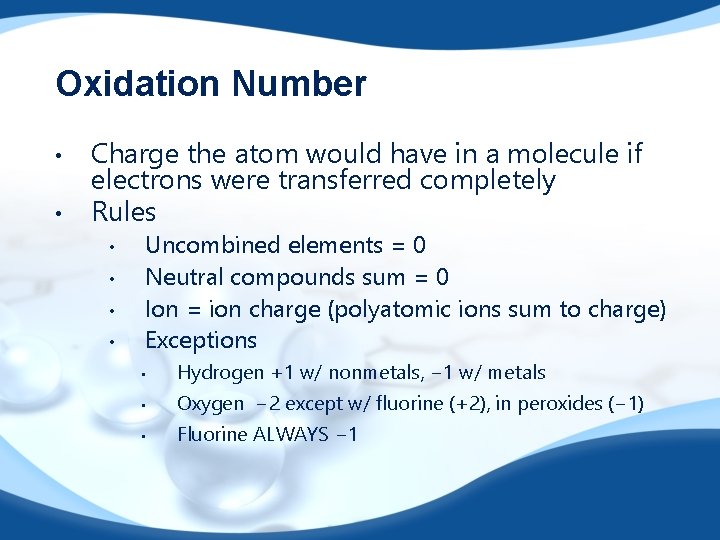
Oxidation Number • • Charge the atom would have in a molecule if electrons were transferred completely Rules • • Uncombined elements = 0 Neutral compounds sum = 0 Ion = ion charge (polyatomic ions sum to charge) Exceptions • Hydrogen +1 w/ nonmetals, − 1 w/ metals • Oxygen − 2 except w/ fluorine (+2), in peroxides (− 1) • Fluorine ALWAYS − 1

Example: 4. 5 Assign oxidation numbers to all the elements in the following compounds and ion: (a) Li 2 O (b) HNO 3 (c)

More common oxidation numbers are in red.

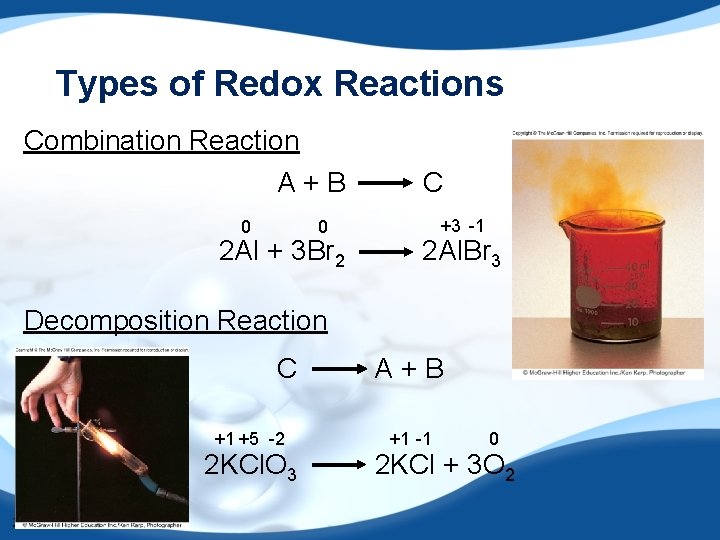
Types of Redox Reactions Combination Reaction A+B 0 0 2 Al + 3 Br 2 C +3 -1 2 Al. Br 3 Decomposition Reaction C +1 +5 -2 2 KCl. O 3 39 A+B +1 -1 0 2 KCl + 3 O 2

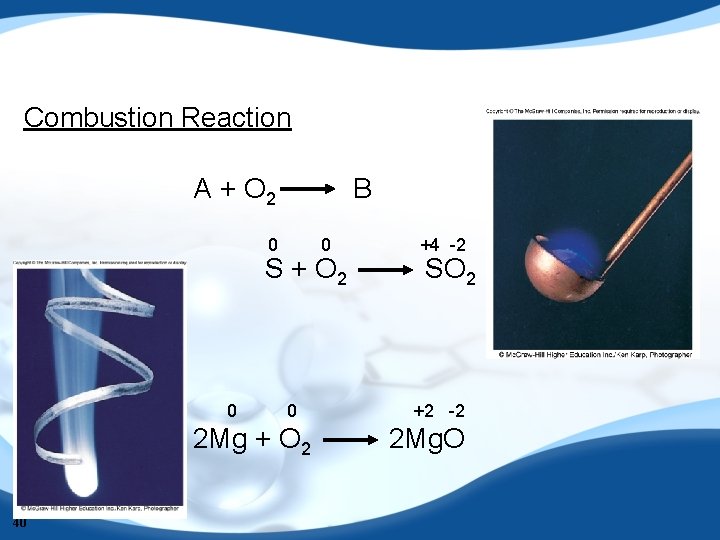
Combustion Reaction A + O 2 B 0 0 S + O 2 0 0 2 Mg + O 2 40 +4 -2 SO 2 +2 -2 2 Mg. O

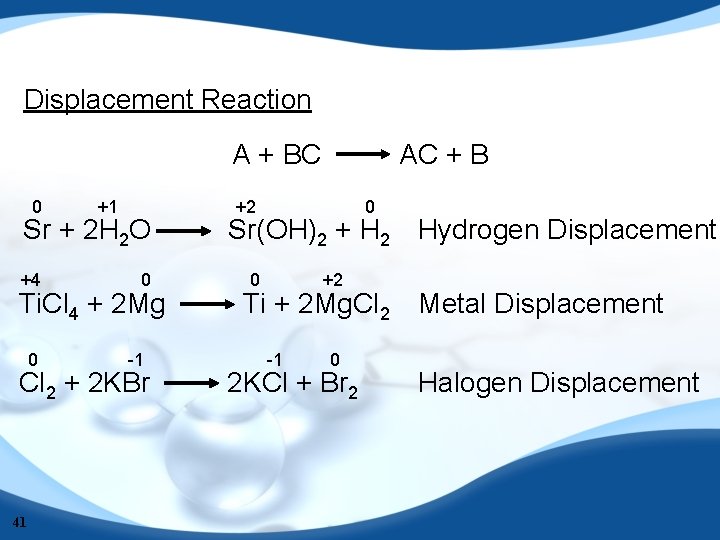
Displacement Reaction A + BC 0 +1 Sr + 2 H 2 O +4 0 Ti. Cl 4 + 2 Mg 0 -1 Cl 2 + 2 KBr 41 AC + B +2 0 Sr(OH)2 + H 2 0 +2 Ti + 2 Mg. Cl 2 -1 0 2 KCl + Br 2 Hydrogen Displacement Metal Displacement Halogen Displacement

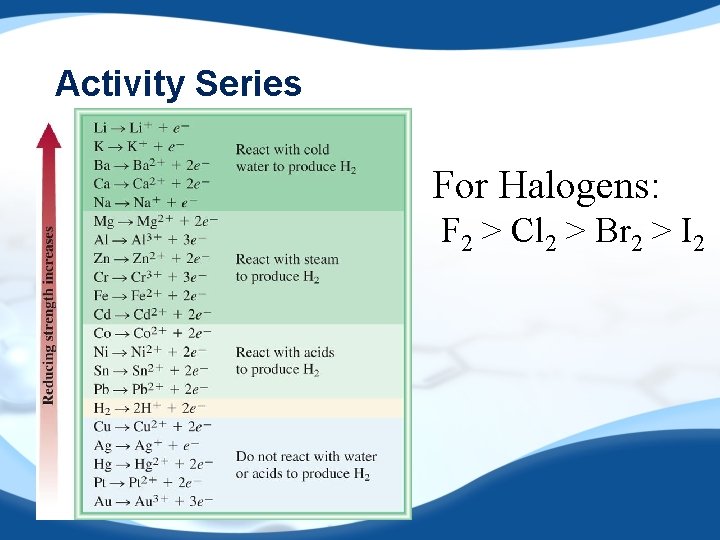
Activity Series For Halogens: F 2 > Cl 2 > Br 2 > I 2

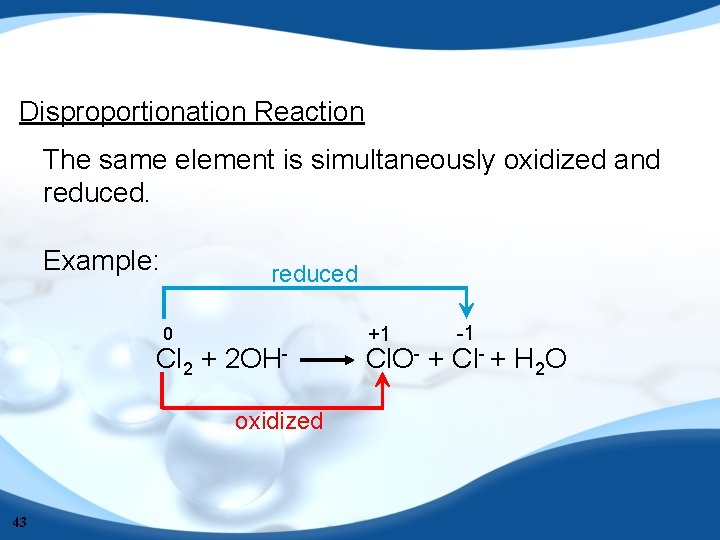
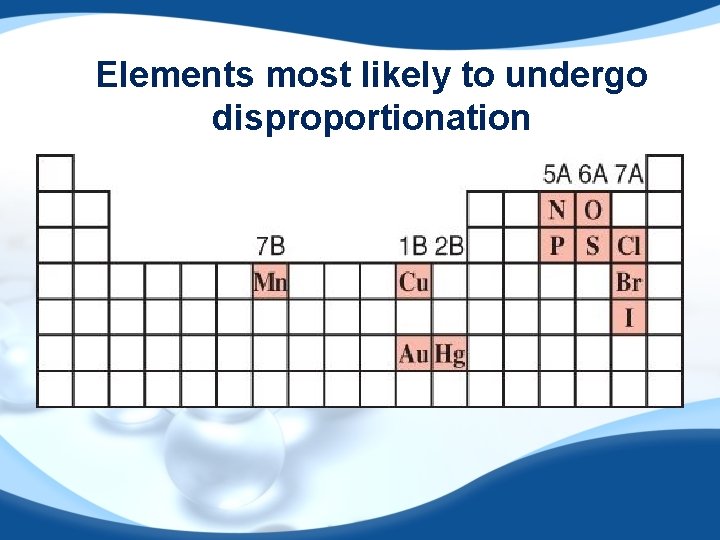
Disproportionation Reaction The same element is simultaneously oxidized and reduced. Example: reduced 0 Cl 2 + 2 OHoxidized 43 +1 -1 Cl. O- + Cl- + H 2 O

Elements most likely to undergo disproportionation

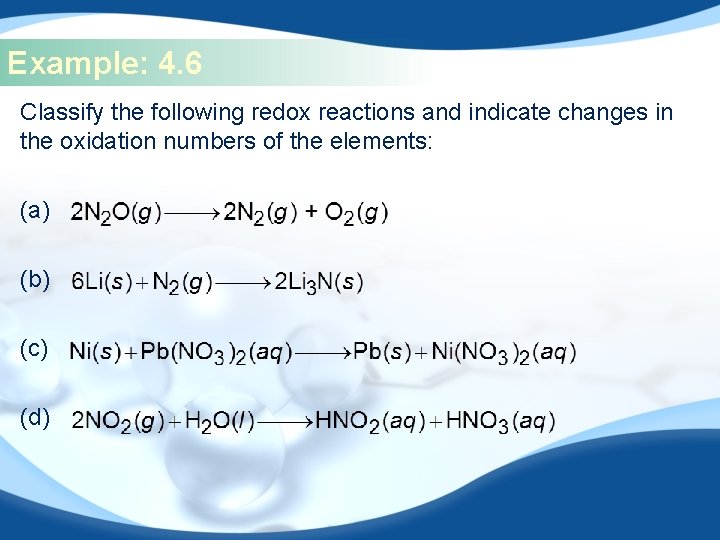
Example: 4. 6 Classify the following redox reactions and indicate changes in the oxidation numbers of the elements: (a) (b) (c) (d)

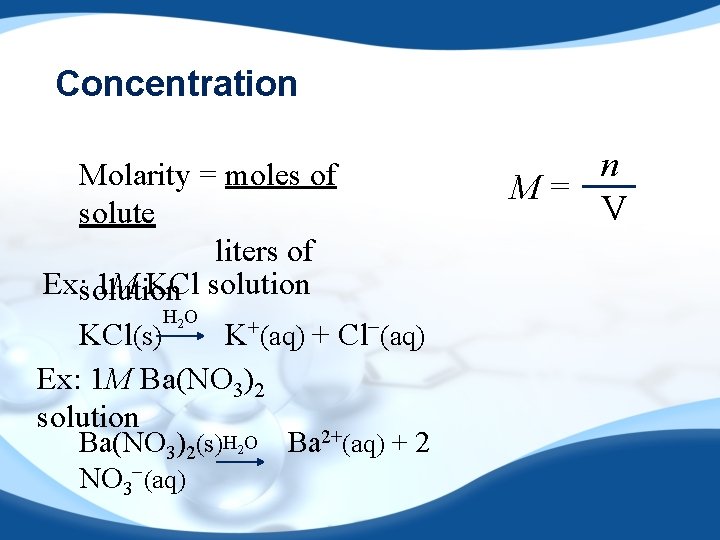
Concentration Molarity = moles of solute liters of Ex: solution 1 M KCl solution H 2 O KCl(s) K+(aq) + Cl−(aq) Ex: 1 M Ba(NO 3)2 solution Ba(NO 3)2(s)H 2 O Ba 2+(aq) + 2 NO 3−(aq) n M= V

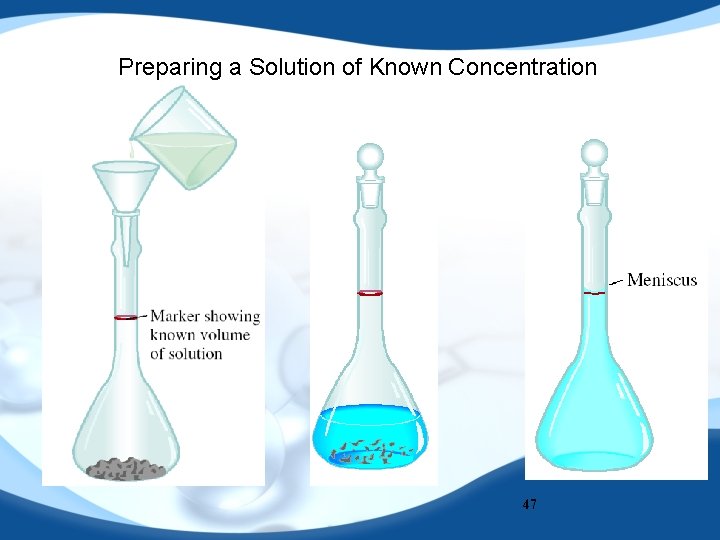
Preparing a Solution of Known Concentration 47

Example: 4. 7 How many grams of potassium dichromate (K 2 Cr 2 O 7) are required to prepare a 250 -m. L solution whose concentration is 2. 16 M? A K 2 Cr 2 O 7 solution.

Example: 4. 8 In a biochemical assay, a chemist needs to add 3. 81 g of glucose to a reaction mixture. Calculate the volume in milliliters of a 2. 53 M glucose solution she should use for the addition.

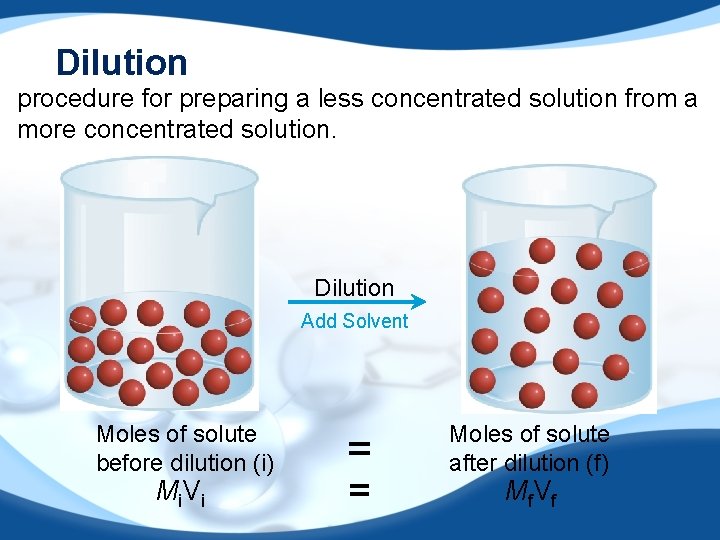
Dilution procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) Mi V i = = Moles of solute after dilution (f) Mf V f

Example: 4. 9 Describe how you would prepare 5. 00 × 102 m. L of a 1. 75 M H 2 SO 4 solution, starting with an 8. 61 M stock solution of H 2 SO 4.

Review of Concepts What is the final concentration of a 0. 6 M Na. Cl solution if its volume is doubled and the number of moles of solute is tripled?

Quantitative analysis • • Gravimetric analysis Titrations • • Acid-base redox

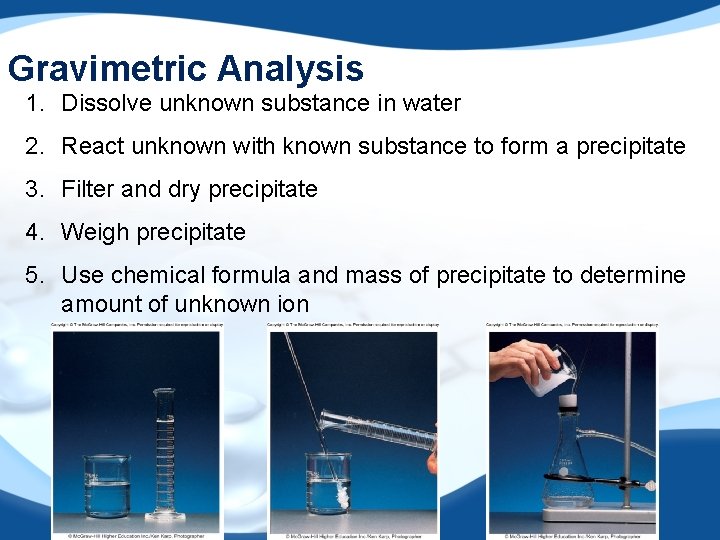
Gravimetric Analysis 1. Dissolve unknown substance in water 2. React unknown with known substance to form a precipitate 3. Filter and dry precipitate 4. Weigh precipitate 5. Use chemical formula and mass of precipitate to determine amount of unknown ion

Example: 4. 10 A 0. 5662 -g sample of an ionic compound containing chloride ions and an unknown metal is dissolved in water and treated with an excess of Ag. NO 3. If 1. 0882 g of Ag. Cl precipitate forms, what is the percent by mass of Cl in the original compound?

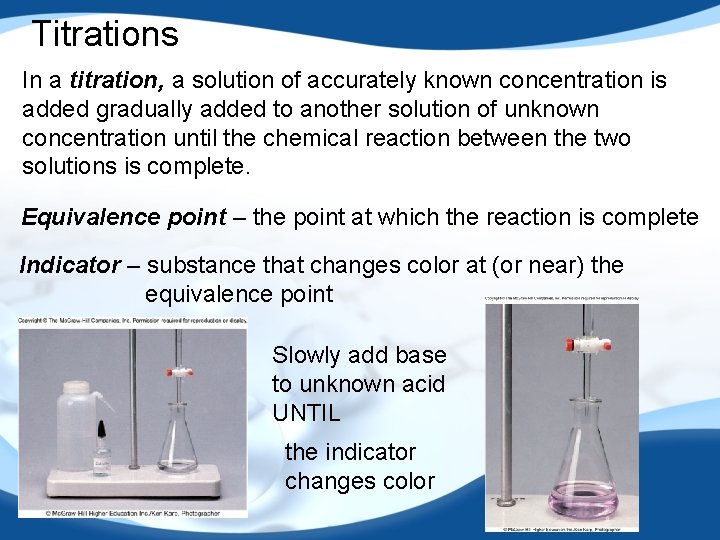
Titrations In a titration, a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the equivalence point Slowly add base to unknown acid UNTIL the indicator changes color 56

Titrations can be used in the analysis of Acid-base reactions H 2 SO 4 + 2 Na. OH 2 H 2 O + Na 2 SO 4 Redox reactions 5 Fe 2+ + Mn. O 4 - + 8 H+ 57 Mn 2+ + 5 Fe 3+ + 4 H 2 O

Example: 4. 11 In a titration experiment, a student finds that 23. 48 m. L of a Na. OH solution are needed to neutralize 0. 5468 g of KHP. What is the concentration (in molarity) of the Na. OH solution?

Example: 4. 12 How many milliliters (m. L) of a 0. 610 M Na. OH solution are needed to neutralize 20. 0 m. L of a 0. 245 M H 2 SO 4 solution?

Redox titrations

Example: 4. 13 A 16. 42 -m. L volume of 0. 1327 M KMn. O 4 solution is needed to oxidize 25. 00 m. L of a Fe. SO 4 solution in an acidic medium. What is the concentration of the Fe. SO 4 solution in molarity? The net ionic equation is