Reactions in Aqueous Solution Chapter 4 4 1






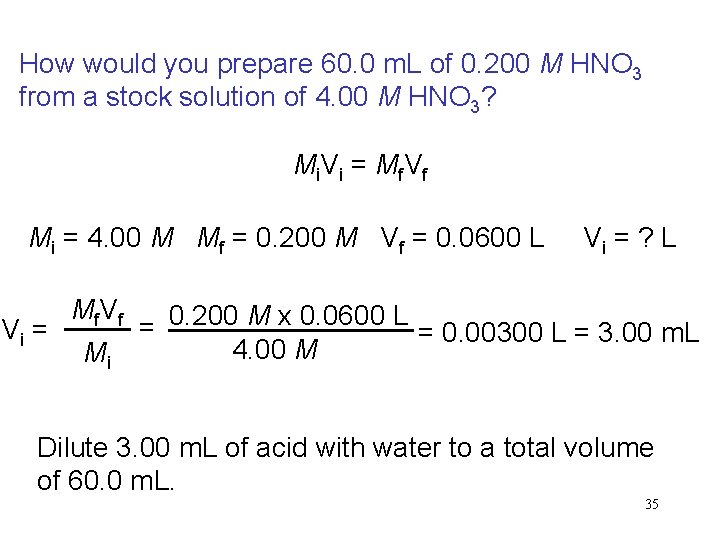

- Slides: 36

Reactions in Aqueous Solution Chapter 4 4. 1 General properties of aqueous solutions 4. 2 Precipitation reactions 4. 4 Oxidation-reduction reactions 4. 5 Concentration of solutions Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

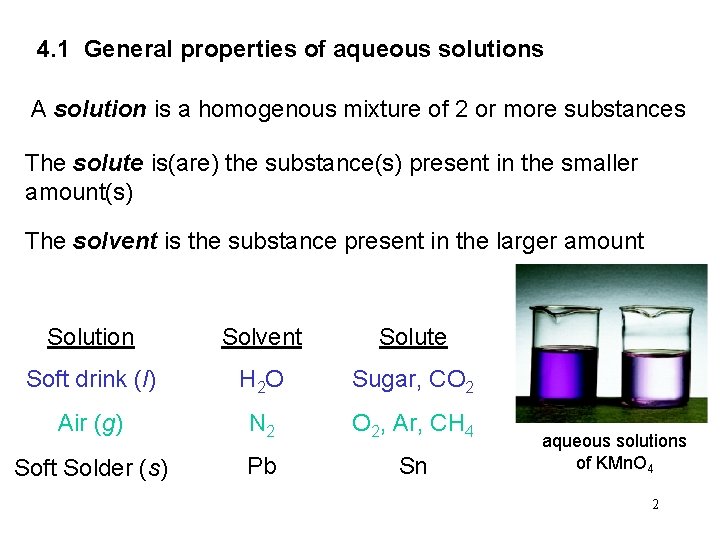
4. 1 General properties of aqueous solutions A solution is a homogenous mixture of 2 or more substances The solute is(are) the substance(s) present in the smaller amount(s) The solvent is the substance present in the larger amount Solution Solvent Solute Soft drink (l) H 2 O Sugar, CO 2 Air (g) N 2 O 2, Ar, CH 4 Soft Solder (s) Pb Sn aqueous solutions of KMn. O 4 2

An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. nonelectrolyte weak electrolyte strong electrolyte 3

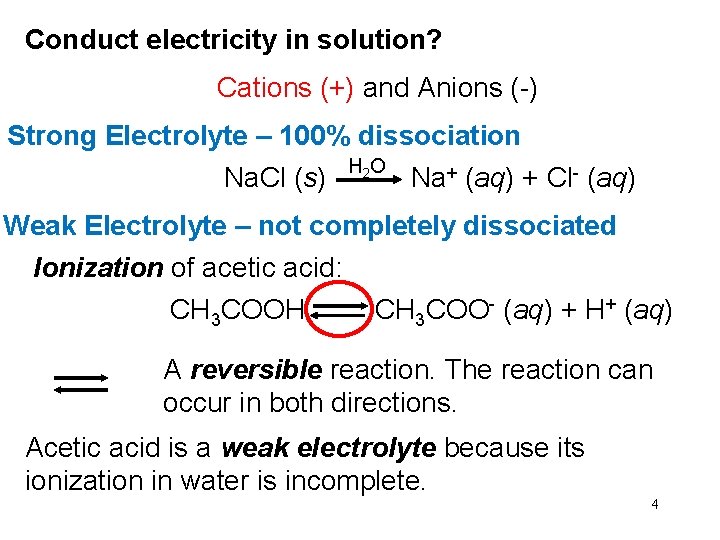
Conduct electricity in solution? Cations (+) and Anions (-) Strong Electrolyte – 100% dissociation Na. Cl (s) H 2 O Na+ (aq) + Cl- (aq) Weak Electrolyte – not completely dissociated Ionization of acetic acid: CH 3 COOH CH 3 COO- (aq) + H+ (aq) A reversible reaction. The reaction can occur in both directions. Acetic acid is a weak electrolyte because its ionization in water is incomplete. 4

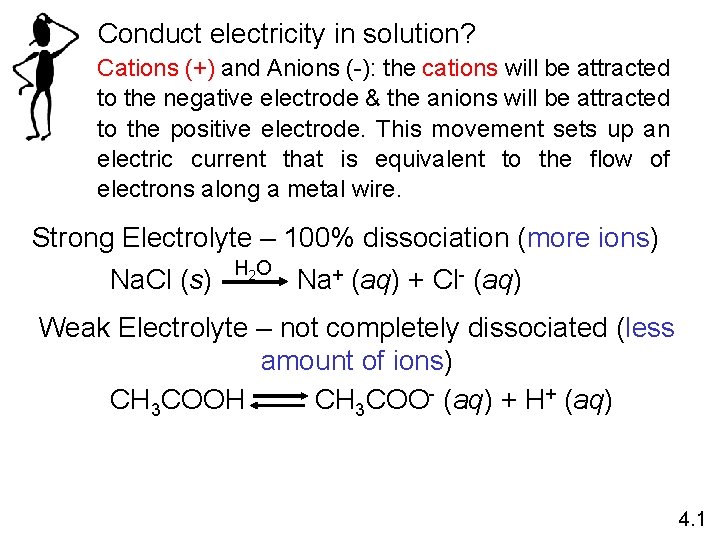
Conduct electricity in solution? Cations (+) and Anions (-): the cations will be attracted to the negative electrode & the anions will be attracted to the positive electrode. This movement sets up an electric current that is equivalent to the flow of electrons along a metal wire. Strong Electrolyte – 100% dissociation (more ions) Na. Cl (s) H 2 O Na+ (aq) + Cl- (aq) Weak Electrolyte – not completely dissociated (less amount of ions) CH 3 COOH CH 3 COO- (aq) + H+ (aq) 4. 1

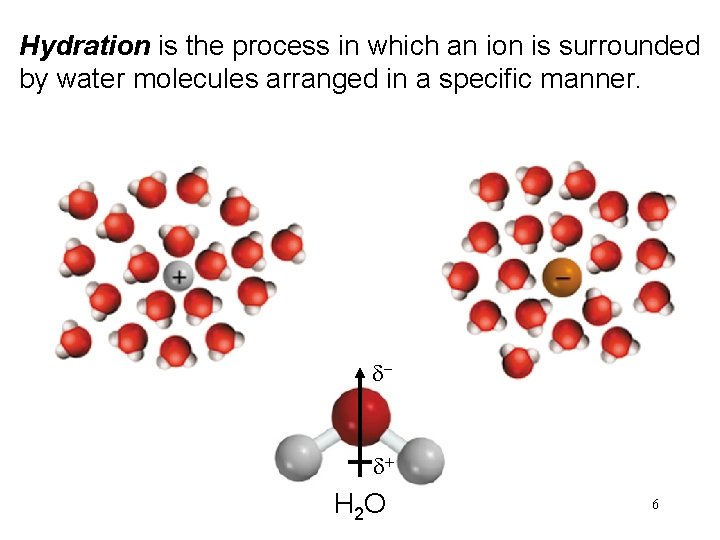
Hydration is the process in which an ion is surrounded by water molecules arranged in a specific manner. d- d+ H 2 O 6

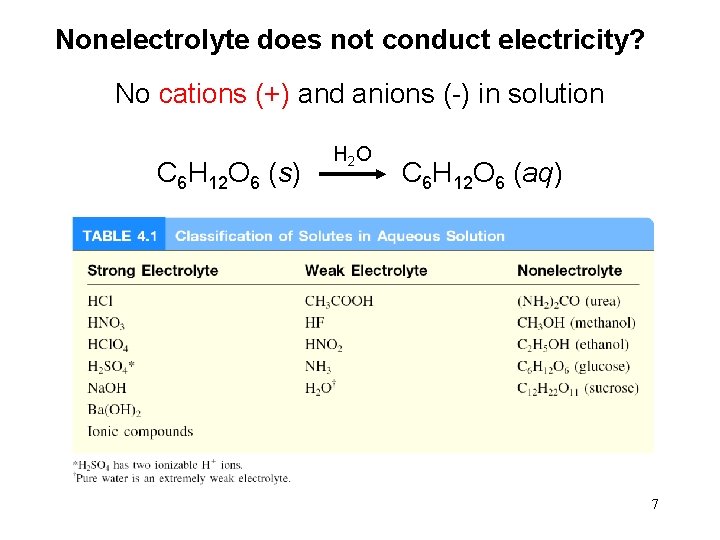
Nonelectrolyte does not conduct electricity? No cations (+) and anions (-) in solution H 2 O C 6 H 12 O 6 (s) C 6 H 12 O 6 (aq) 7

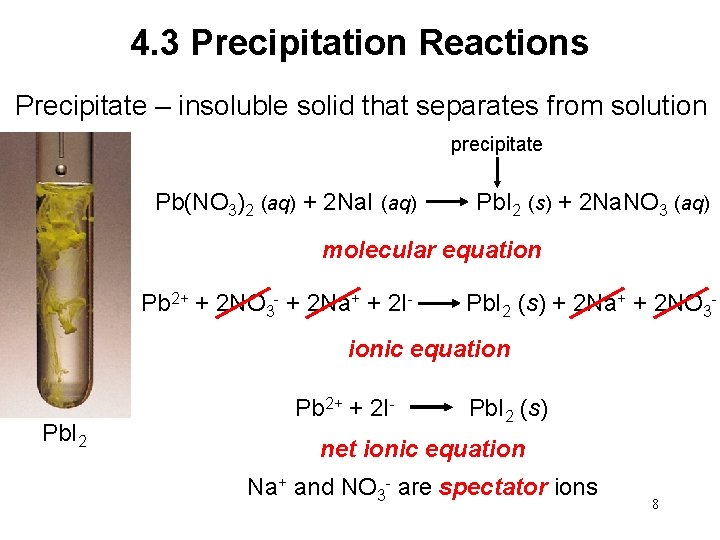
4. 3 Precipitation Reactions Precipitate – insoluble solid that separates from solution precipitate Pb(NO 3)2 (aq) + 2 Na. I (aq) Pb. I 2 (s) + 2 Na. NO 3 (aq) molecular equation Pb 2+ + 2 NO 3 - + 2 Na+ + 2 I- Pb. I 2 (s) + 2 Na+ + 2 NO 3 ionic equation Pb. I 2 Pb 2+ + 2 I- Pb. I 2 (s) net ionic equation Na+ and NO 3 - are spectator ions 8

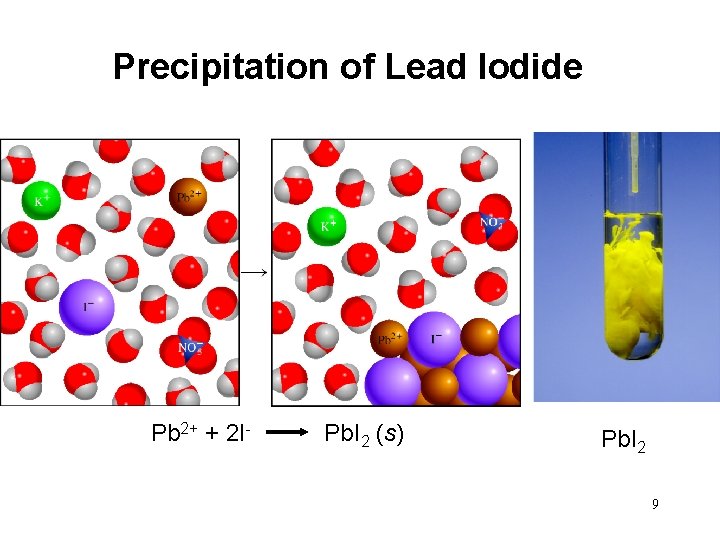
Precipitation of Lead Iodide Pb 2+ + 2 I- Pb. I 2 (s) Pb. I 2 9

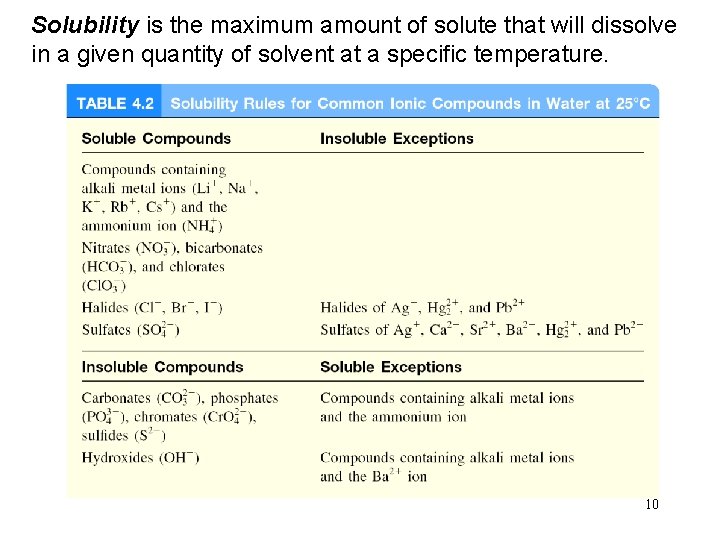
Solubility is the maximum amount of solute that will dissolve in a given quantity of solvent at a specific temperature. 10

Examples of Insoluble Compounds Cd. S Pb. S Ni(OH)2 Al(OH)3 11

Question: Classify the following ionic compounds as ‘soluble’ or ‘insoluble’ in water: Answer: 1. Na 2 CO 3 2. Pb. SO 4 3. Co(OH)2 4. Ba(NO 3)2 5. (NH 4)3 PO 4 12

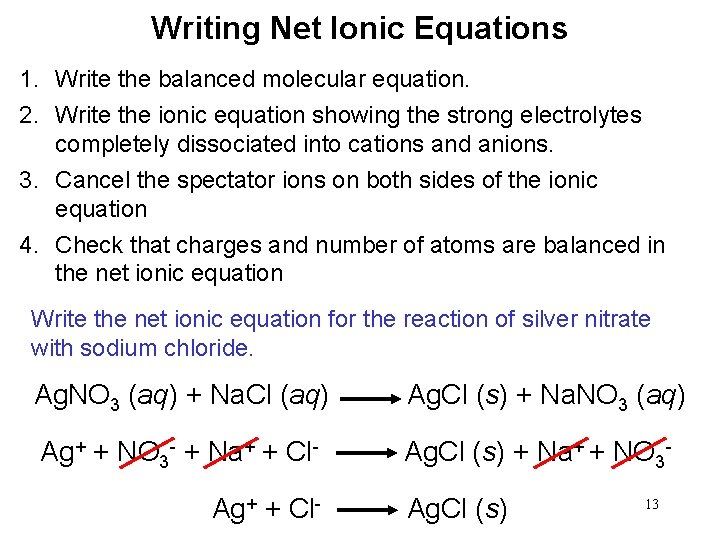
Writing Net Ionic Equations 1. Write the balanced molecular equation. 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation 4. Check that charges and number of atoms are balanced in the net ionic equation Write the net ionic equation for the reaction of silver nitrate with sodium chloride. Ag. NO 3 (aq) + Na. Cl (aq) Ag. Cl (s) + Na. NO 3 (aq) Ag+ + NO 3 - + Na+ + Cl- Ag. Cl (s) + Na+ + NO 3 - Ag+ + Cl- Ag. Cl (s) 13

Question: Write the net ionic equation for the precipitation reaction that occurs when solution of calcium chloride and sodium carbonate are mixed. 14

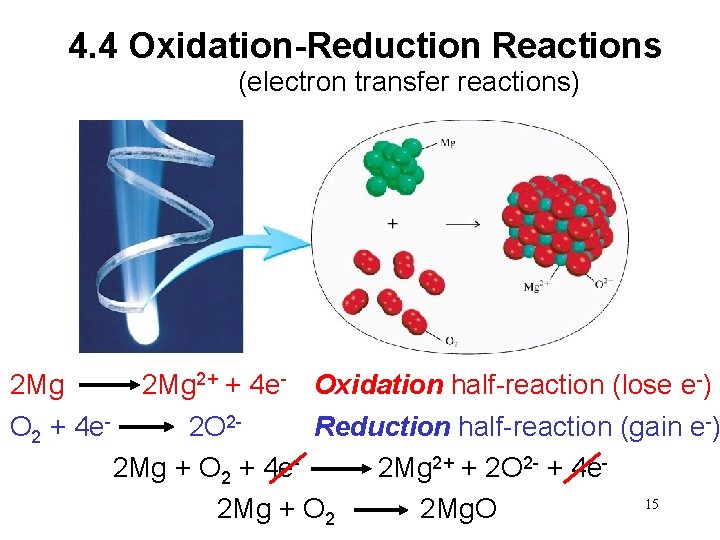
4. 4 Oxidation-Reduction Reactions (electron transfer reactions) 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) O 2 + 4 e- 2 O 2 Reduction half-reaction (gain e-) 2 Mg + O 2 + 4 e- 2 Mg 2+ + 2 O 2 - + 4 e 15 2 Mg + O 2 2 Mg. O

16

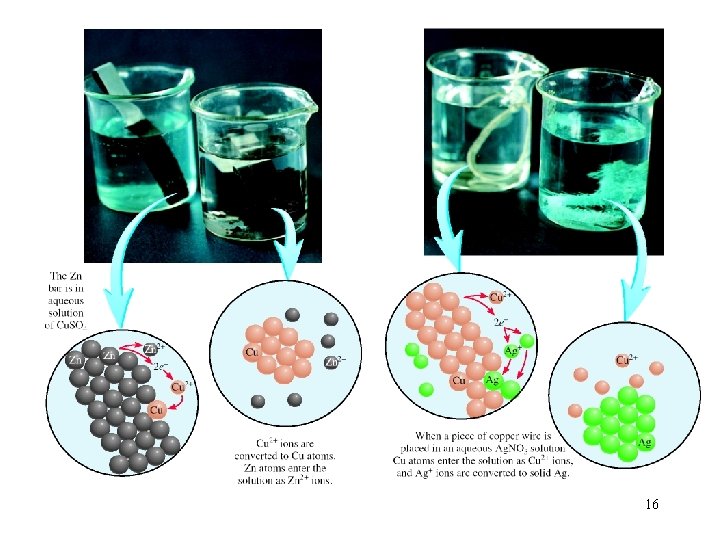
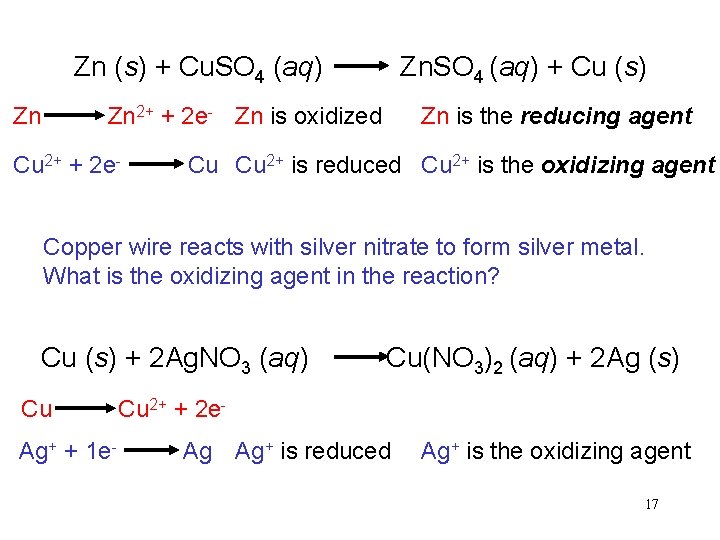
Zn (s) + Cu. SO 4 (aq) Zn. SO 4 (aq) + Cu (s) Zn 2+ + 2 e- Zn is oxidized Zn is the reducing agent Cu 2+ + 2 e- Cu Cu 2+ is reduced Cu 2+ is the oxidizing agent Copper wire reacts with silver nitrate to form silver metal. What is the oxidizing agent in the reaction? Cu (s) + 2 Ag. NO 3 (aq) Cu(NO 3)2 (aq) + 2 Ag (s) Cu 2+ + 2 e. Ag+ + 1 e- Ag Ag+ is reduced Ag+ is the oxidizing agent 17

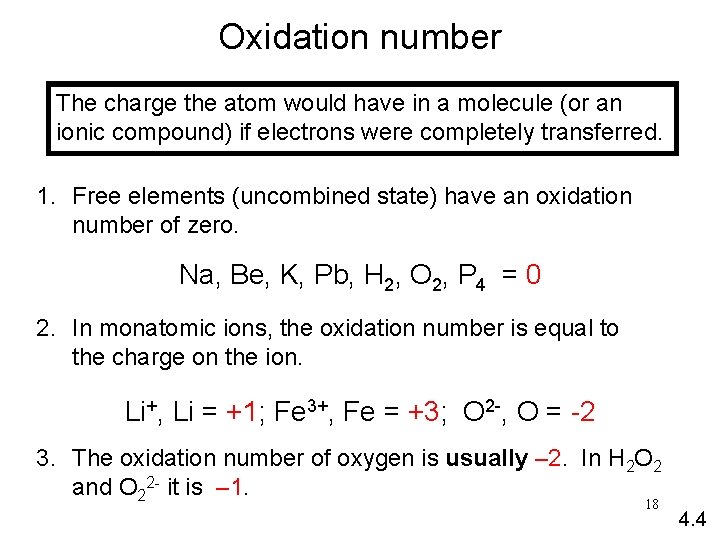
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2. In H 2 O 2 and O 22 - it is – 1. 18 4. 4

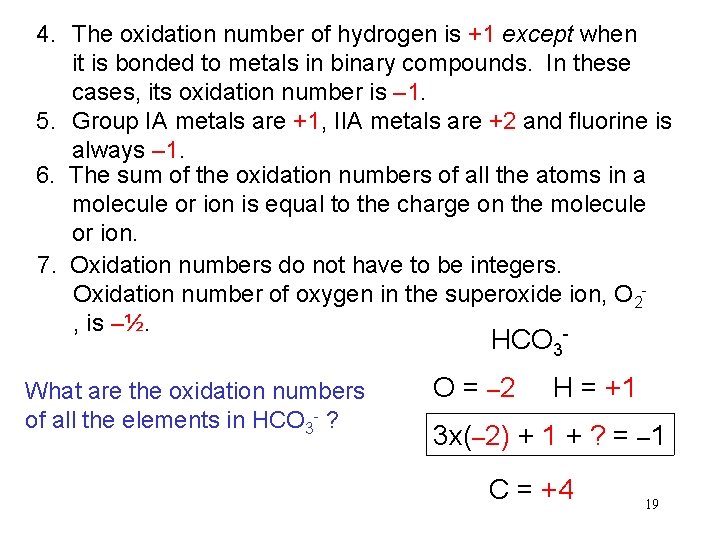
4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is – 1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always – 1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O 2, is –½. - HCO 3 What are the oxidation numbers of all the elements in HCO 3 - ? O = – 2 H = +1 3 x(– 2) + 1 + ? = – 1 C = +4 19

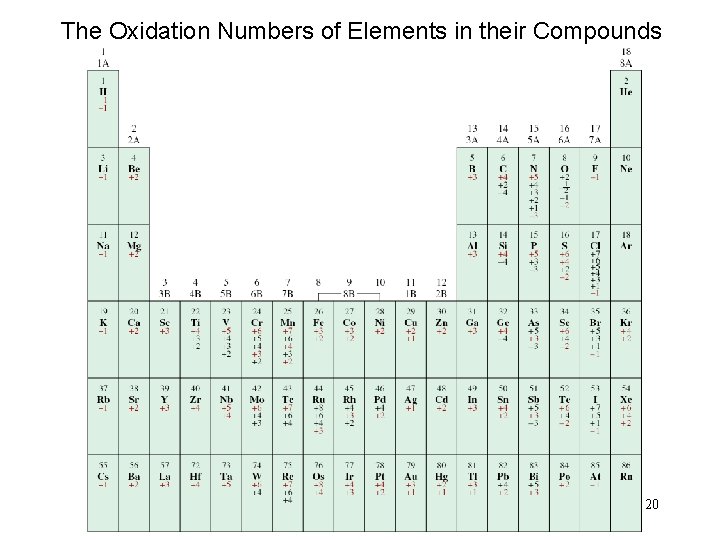
The Oxidation Numbers of Elements in their Compounds 20

What are the oxidation numbers of all the elements in each of these compounds? Na. IO 3 IF 7 K 2 Cr 2 O 7 21

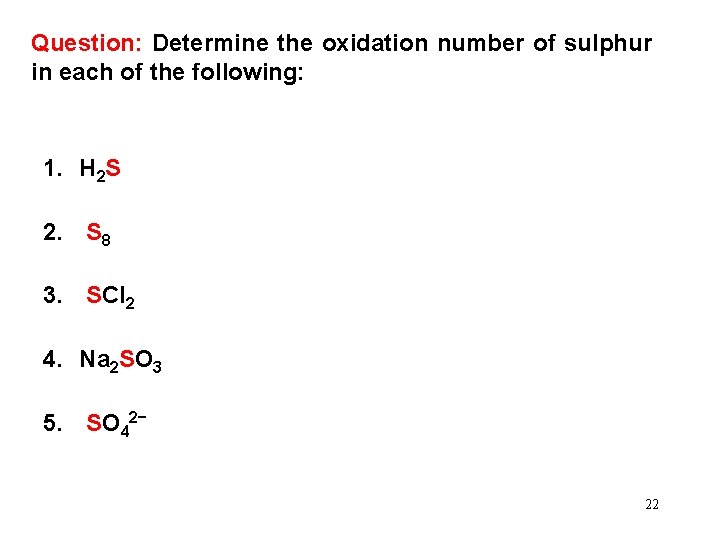
Question: Determine the oxidation number of sulphur in each of the following: 1. H 2 S 2. S 8 3. SCl 2 4. Na 2 SO 3 5. SO 42− 22

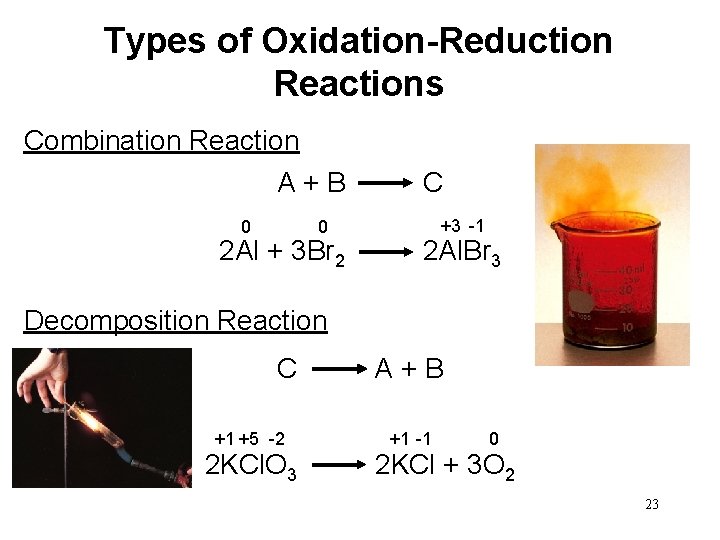
Types of Oxidation-Reduction Reactions Combination Reaction A + B C 0 +3 -1 0 2 Al + 3 Br 2 2 Al. Br 3 Decomposition Reaction C A + B +1 +5 -2 +1 -1 0 2 KCl. O 3 2 KCl + 3 O 2 23

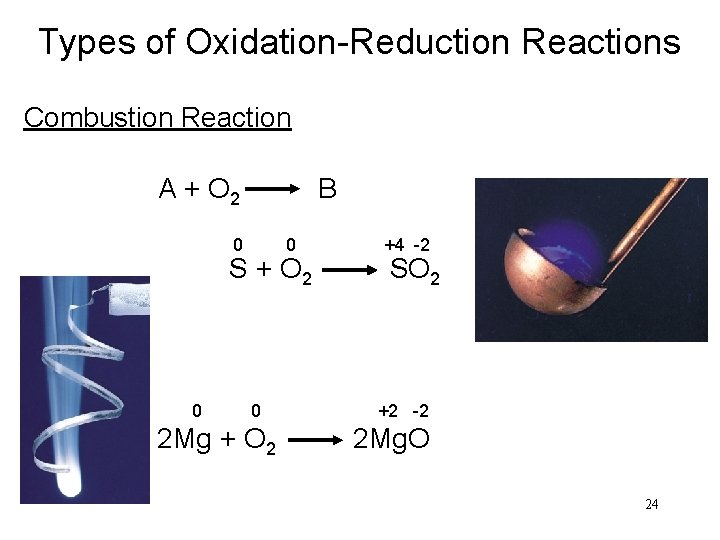
Types of Oxidation-Reduction Reactions Combustion Reaction A + O 2 B 0 0 +4 -2 S + O 2 SO 2 0 0 +2 -2 2 Mg + O 2 2 Mg. O 24

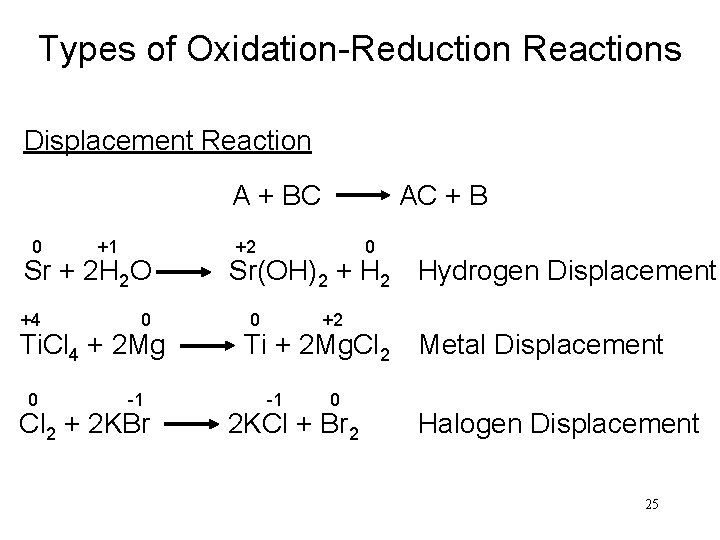
Types of Oxidation-Reduction Reactions Displacement Reaction A + BC AC + B 0 +1 +2 0 Sr + 2 H 2 O Sr(OH)2 + H 2 Hydrogen Displacement +4 0 0 +2 Ti. Cl 4 + 2 Mg Ti + 2 Mg. Cl 2 Metal Displacement 0 -1 -1 0 Cl 2 + 2 KBr 2 KCl + Br 2 Halogen Displacement 25

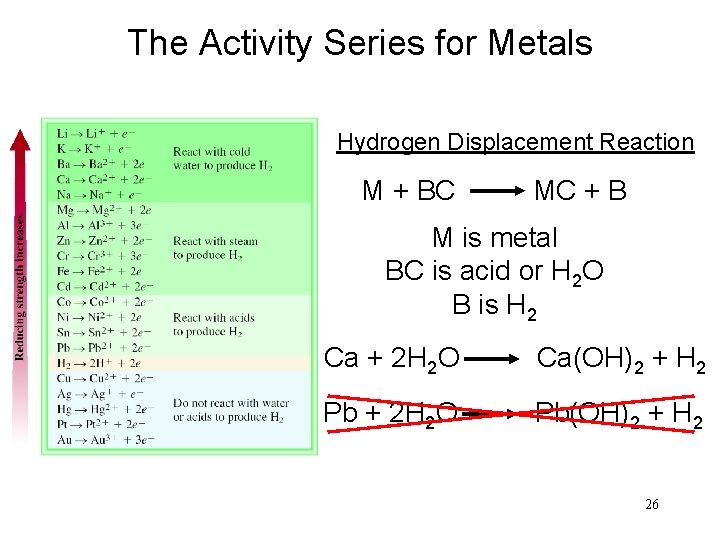
The Activity Series for Metals Hydrogen Displacement Reaction M + BC MC + B M is metal BC is acid or H 2 O B is H 2 Ca + 2 H 2 O Ca(OH)2 + H 2 Pb + 2 H 2 O Pb(OH)2 + H 2 26

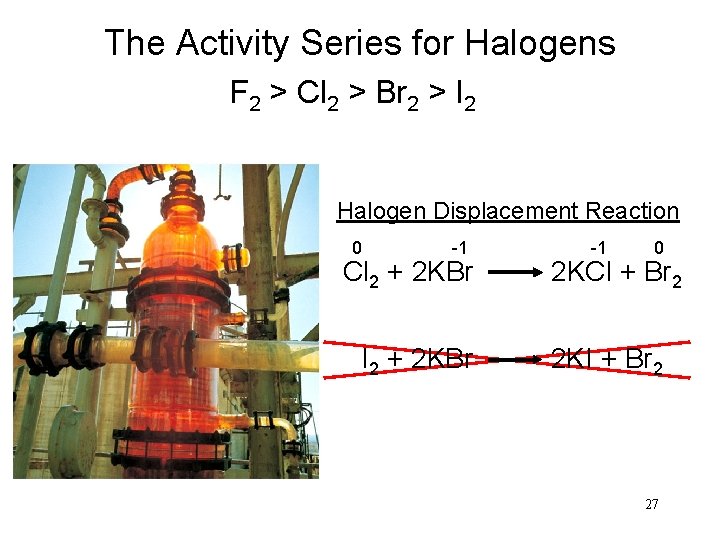
The Activity Series for Halogens F 2 > Cl 2 > Br 2 > I 2 Halogen Displacement Reaction 0 -1 -1 0 Cl 2 + 2 KBr 2 KCl + Br 2 I 2 + 2 KBr 2 KI + Br 2 27

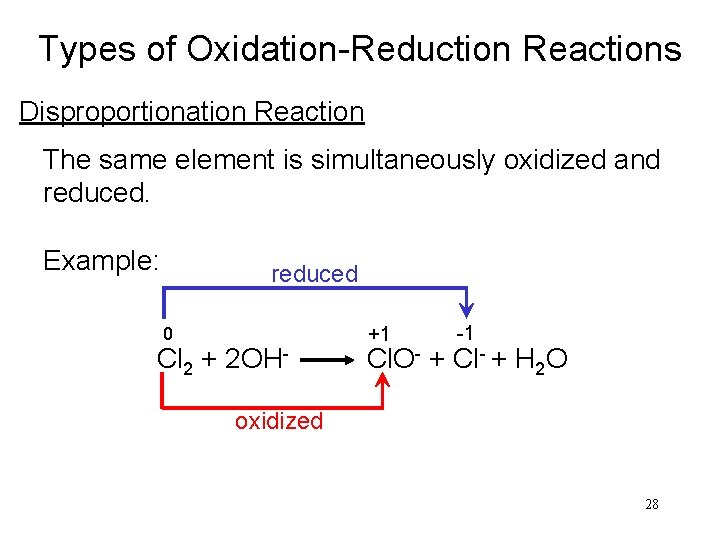
Types of Oxidation-Reduction Reactions Disproportionation Reaction The same element is simultaneously oxidized and reduced. Example: reduced +1 0 -1 Cl 2 + 2 OH- Cl. O- + Cl- + H 2 O oxidized 28

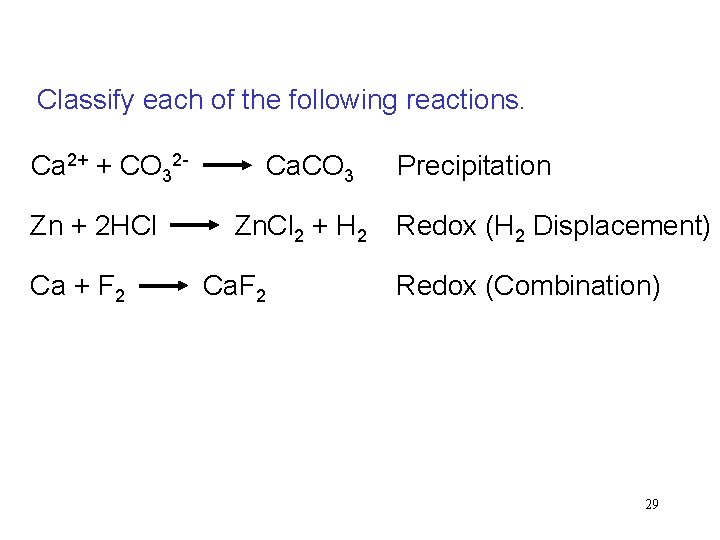
Classify each of the following reactions. Ca 2+ + CO 32 - Ca. CO 3 Precipitation Zn + 2 HCl Zn. Cl 2 + H 2 Redox (H 2 Displacement) Ca + F 2 Ca. F 2 Redox (Combination) 29

Question: Classify the following reactions. For redox reaction, identify the oxidizing and reducing agents. 1. Barium chloride(aq) and ammonium sulfate(aq) 2. Manganese metal and tin IV chloride (aq) 3. Cobalt metal and nitrogen gas to yield cobalt (II) nitride 4. Sodium peroxide to give sodium oxide and oxygen gas

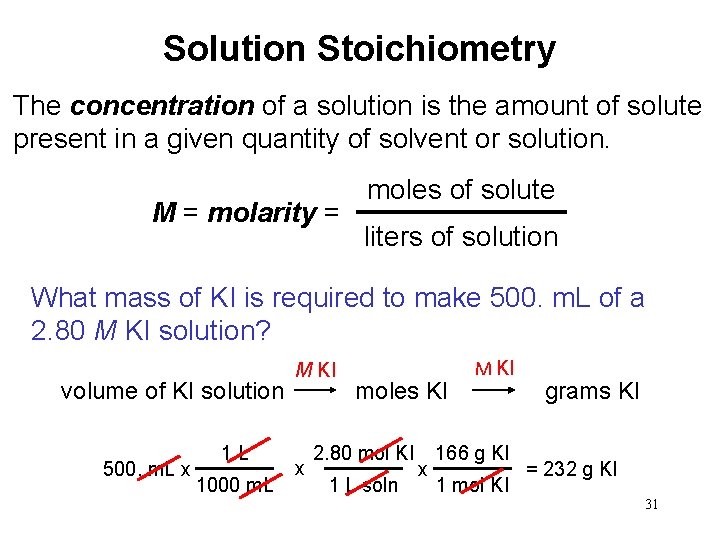
Solution Stoichiometry The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. M = molarity = moles of solute liters of solution What mass of KI is required to make 500. m. L of a 2. 80 M KI solution? volume of KI solution 500. m. L x 1 L 1000 m. L M KI x moles KI 2. 80 mol KI 1 L soln x M KI 166 g KI 1 mol KI grams KI = 232 g KI 31

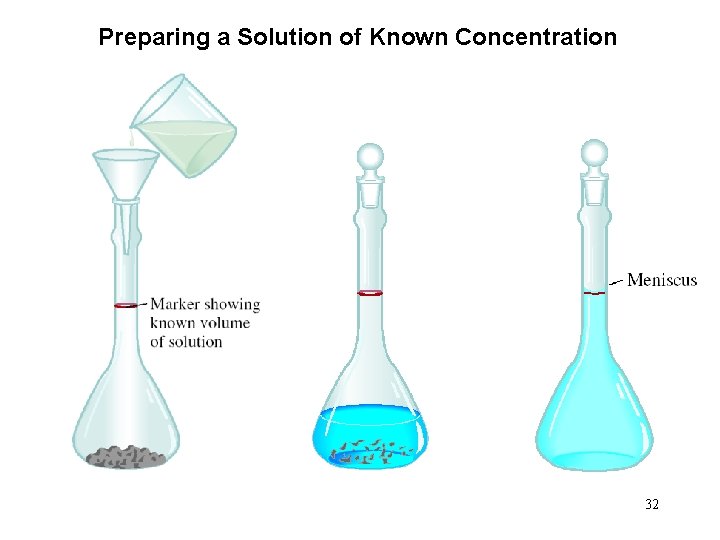
Preparing a Solution of Known Concentration 32

Question: Calculate the molarity of a solution made by dissolving 23. 4 g of sodium sulphate in enough water to form 125 m. L of solution. Question: What are the molar concentrations of each of the ions present in a 0. 025 M aqueous solution of calcium nitrate? Question: How many grams of Na 2 SO 4 are required to make 0. 350 L of 0. 500 M aqueous solution of Na 2 SO 4? 33

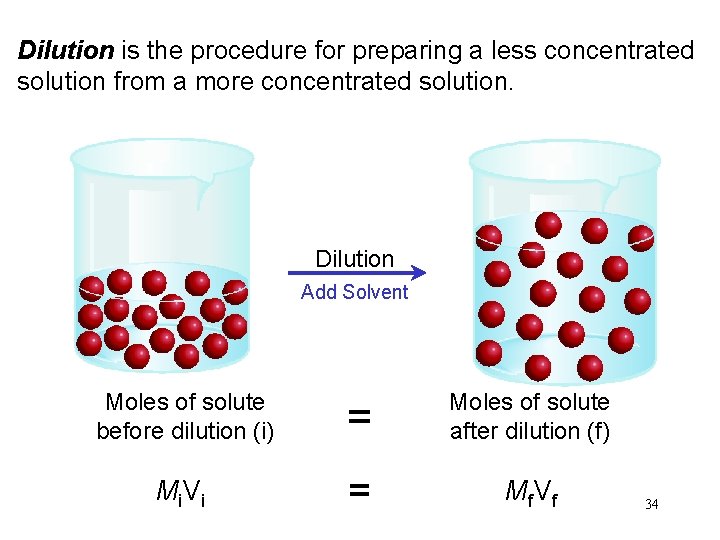
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) = Moles of solute after dilution (f) Mi V i = Mf V f 34
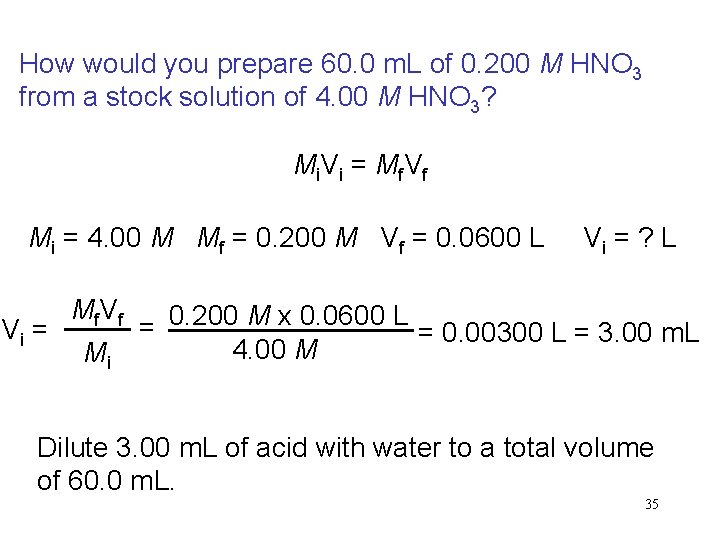
How would you prepare 60. 0 m. L of 0. 200 M HNO 3 from a stock solution of 4. 00 M HNO 3? Mi. Vi = Mf. Vf Mi = 4. 00 M Mf = 0. 200 M Vf = 0. 0600 L Vi = Mf V f Mi Vi = ? L = 0. 200 M x 0. 0600 L = 0. 00300 L = 3. 00 m. L 4. 00 M Dilute 3. 00 m. L of acid with water to a total volume of 60. 0 m. L. 35

Question: How many m. L of 3. 0 M H 2 SO 4 are needed to make 450 m. L of 0. 10 M H 2 SO 4? Question: If 10. 0 m. L of a 10. 0 M stock solution of Na. OH is diluted to 250 m. L, what is the concentration of the resulting solution? 36