Reactions Chemistry Odds Ends Organics Atoms 100 100



















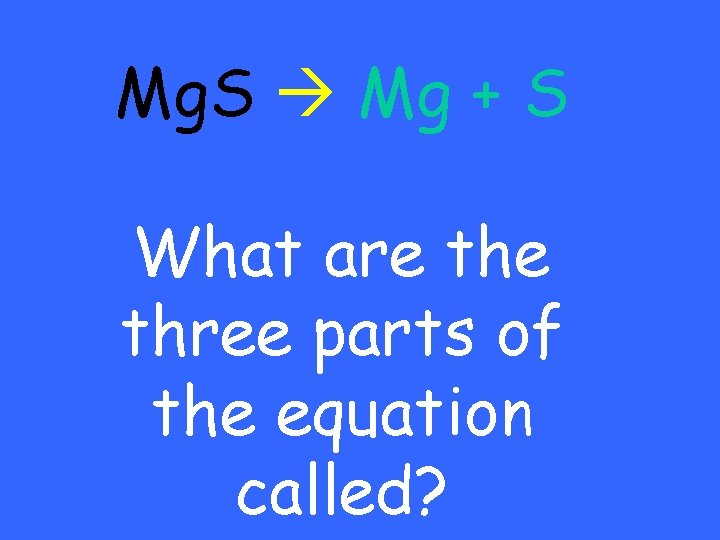

























- Slides: 52

Reactions Chemistry Odds & Ends Organics Atoms 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

Why is carbon so important?

It is part of every living thing • DNA • Protein • Carbohydrates

Why can carbon combine with itself as well as other elements?

It has 4 Valence electrons

Organic compounds contain what 6 elements?

Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus and Sulfur

What is the simplest form of an organic compound?

Hydrocarbons

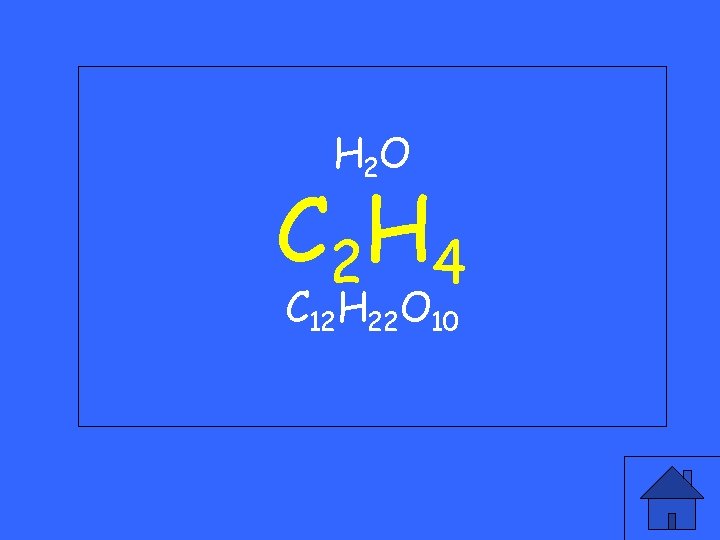
Which of the following is a hydrocarbon? H 2 O C 2 H 4 C 12 H 22 O 10

H 2 O C 2 H 4 C 12 H 22 O 10

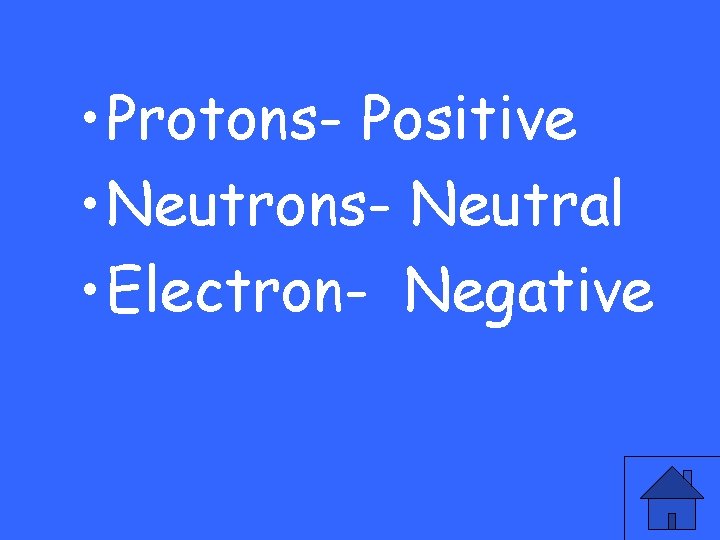
What are the charges of Protons, Neutrons and electrons?

• Protons- Positive • Neutrons- Neutral • Electron- Negative

Which 2 parts of the atom have the most mass?

Protons and Neutrons They make up most of the mass of the atom

Where can Valence electrons be found?

In the outer shell Also known as a electron cloud

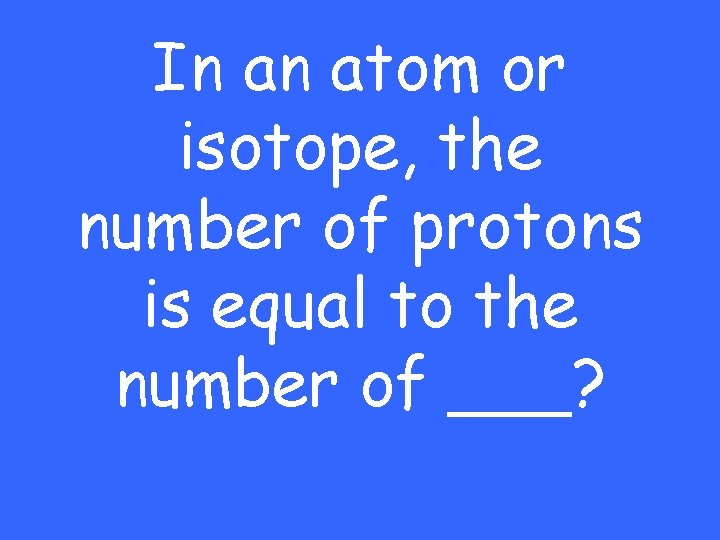
In an atom or isotope, the number of protons is equal to the number of ___?

The number of electrons This changes if an atom is an ion

Ions are?

Positively or negatively charged atoms

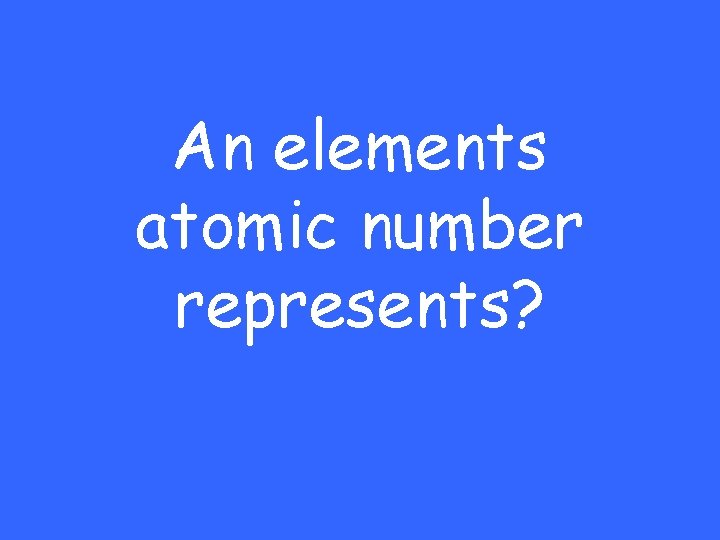
An elements atomic number represents?

The number of Protons
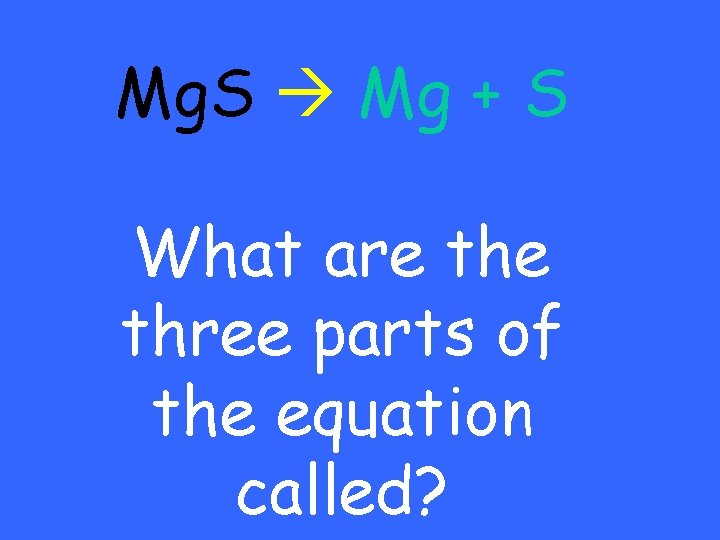
Mg. S Mg + S What are three parts of the equation called?

Products (yield) Reactants

List two types of bonds that atoms can make?

Ionic, Covalent

What is the only thing that changes during a chemical reaction?

The order the atoms are arranged

What type of reaction is cooking a pizza?

Chemical Reaction

What is the Law of Conservation of Mass (in a chemical reaction)?

In a chemical reactions, atoms are neither created or destroyed, merely rearranged

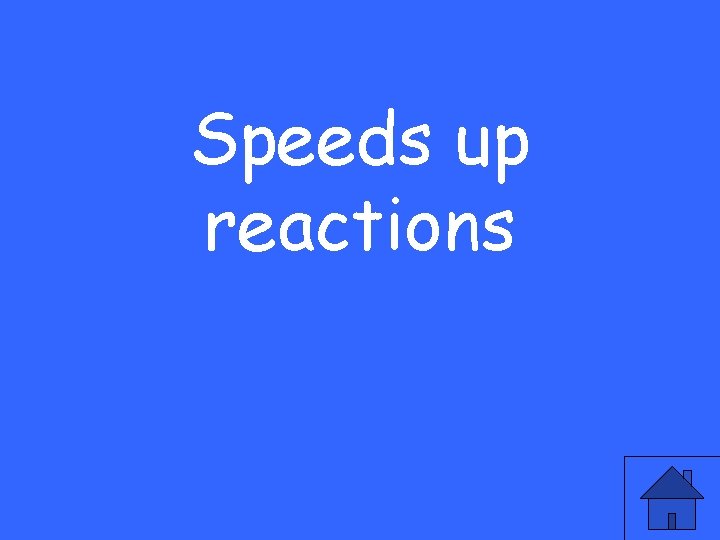
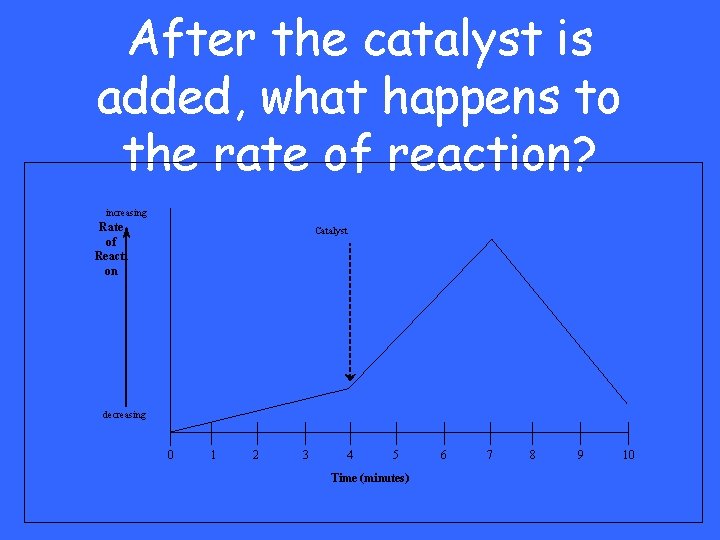
What does a catalyst do?

Speeds up reactions

What is created when a chemical change occurs?

A new substance with new chemical properties is created

How many protons does Fluorine have?

9 Protons

What you start a reaction with and what you end a reaction with. What are these two things called?

Product and Reactant

Force =Mass x Acceleration If the Force is 75 N and the acceleration is 2 10 m/sec

Force =Mass x Acceleration 7. 5 kg x 10 m/sec = 75 N

Force =Mass x Acceleration If the mass is 100 kg and the acceleration is 2 5 m/sec find the force in Newtons?

Force =Mass x Acceleration 100 kg x 5 m/sec = 500 N

Daily Double Draw a linear graph on the whiteboard

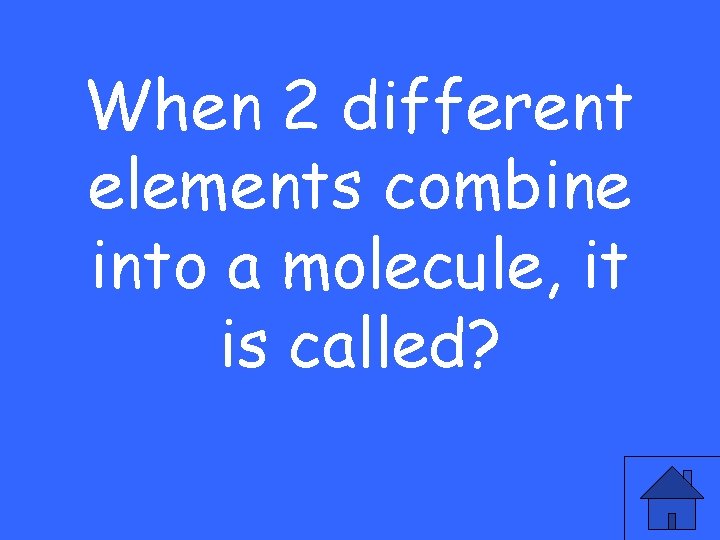
When 2 different elements combine into a molecule, it is called?

A compound

Isotopes have different number of?

Neutrons

After the catalyst is added, what happens to the rate of reaction? increasing Rate of Reacti on Catalyst decreasing 0 1 2 3 4 5 Time (minutes) 6 7 8 9 10

It increases
 100 100 100 100 100
100 100 100 100 100 What kingdom is considered the ods and ends kingdom?
What kingdom is considered the ods and ends kingdom? Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Midwest organics recycling
Midwest organics recycling Regents periodic table
Regents periodic table Rearrangement of atoms in a chemical reaction
Rearrangement of atoms in a chemical reaction In chemical reactions atoms are rearranged
In chemical reactions atoms are rearranged Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Example of oxidation reduction reaction
Example of oxidation reduction reaction Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Types of reactions chemistry
Types of reactions chemistry 5 types of reactions in chemistry
5 types of reactions in chemistry Type of reactions chemistry
Type of reactions chemistry What is the reactant and product
What is the reactant and product Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Slidetodoc.com
Slidetodoc.com Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Cara menghitung attributable risk
Cara menghitung attributable risk Perbedaan relative risk dan odds ratio
Perbedaan relative risk dan odds ratio Gripsholmsstenen
Gripsholmsstenen Rr statistics
Rr statistics Definitions of probability
Definitions of probability Diagnostic odds ratio
Diagnostic odds ratio Odds and evens netball
Odds and evens netball Dimensi penelitian kualitatif
Dimensi penelitian kualitatif Odds ratio interprétation
Odds ratio interprétation Odds ratio interprétation
Odds ratio interprétation Odds ratio interprétation
Odds ratio interprétation Haskell odd
Haskell odd Contoh teori probabilitas
Contoh teori probabilitas Odds ratio
Odds ratio Or
Or Calcular odds ratio
Calcular odds ratio Rule of odds
Rule of odds Haskell odds
Haskell odds The standard days method
The standard days method Nna odds
Nna odds Odds ratio
Odds ratio Study types in medical research
Study types in medical research Dice odds tft
Dice odds tft Tft odds calculator
Tft odds calculator Odds ratio
Odds ratio Skema case control
Skema case control Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Lesson 5 world war 2 ends
Lesson 5 world war 2 ends Lesson 4 world war 1 ends
Lesson 4 world war 1 ends A solid object with two identical ends and flat sides
A solid object with two identical ends and flat sides Character traits of romeo
Character traits of romeo Idiophone instruments in philippines
Idiophone instruments in philippines