Reaction Types Learning Target 1 I can read






- Slides: 22

Reaction Types

Learning Target 1 �I can read and understand the information contained within a chemical reaction

Introduction Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. In a given reaction …. Symbols represent elements (H) Chemical formulas describe compounds (Al 2 O 3)

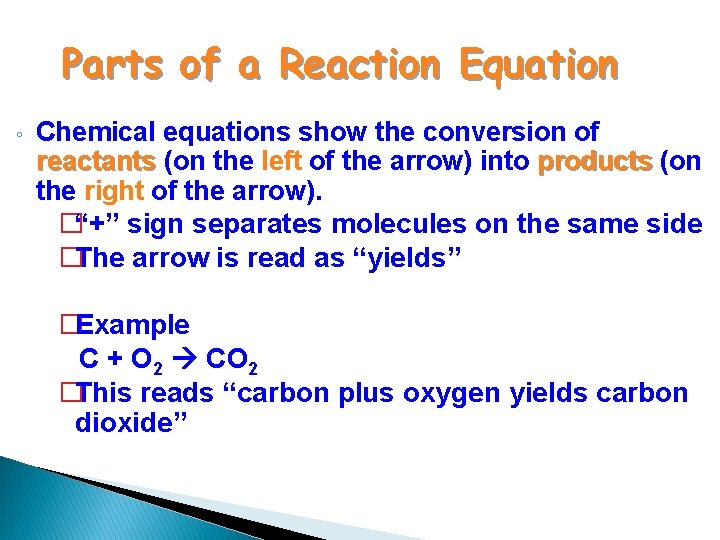
Parts of a Reaction Equation ◦ Chemical equations show the conversion of reactants (on the left of the arrow) into products (on the right of the arrow). �“+” sign separates molecules on the same side �The arrow is read as “yields” �Example C + O 2 CO 2 �This reads “carbon plus oxygen yields carbon dioxide”

The charcoal used in a grill is basically carbon. The carbon reacts with oxygen to yield carbon dioxide. The chemical equation for this reaction, C + O 2 CO 2, contains the same information as the English sentence but has quantitative (numerical) meaning as well.

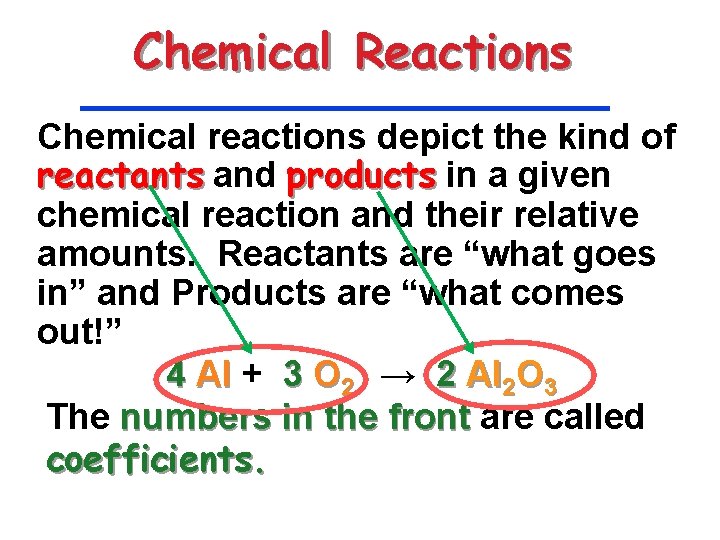
Chemical Reactions Chemical reactions depict the kind of reactants and products in a given chemical reaction and their relative amounts. Reactants are “what goes in” and Products are “what comes out!” 4 Al + 3 O 2 → 2 Al 2 O 3 The numbers in the front are called coefficients.

What coefficients mean … � The coefficient in front of a given element in an equation can stand for a representative particle (one piece) of the substance OR a mole of the substance 4 Al + 3 O 2 ---> 2 Al 2 O 3 4 atoms of aluminum plus 3 molecules of oxygen gas yield two molecules of aluminum oxide OR 4 moles of aluminum plus 3 moles of oxygen gas yield two moles of aluminum oxide

Other Reaction Symbols � (l) “liquid”; this indicates that a chemical is in liquid form. EX: H 2 O(l) � (aq) “aqueous”; this indicates that a chemical is EX: Na. Cl � (g) dissolved. (aq) “gaseous”; this indicates that a chemical is in gaseous form. EX: CO 2(g) � (s) “solid”; this indicates that a chemical is in solid form. EX: Mg(OH)2(s) � “precipitate”; this indicates that a product is a solid precipitate. EX: Ca. CO 3 � “delta” or “heat”; this symbol over the yields sign indicates that heat is added to move the reaction along. . EX: � ↑ “gas”; this indicates that a product is formed which is a gas, and forms bubbles. EX: H 2↑

Learning Target 2 �I can differentiate between the different types of chemical reactions.

Classifying Reactions 〉 〉 How does learning about reaction types help in understanding chemical reactions? You can use patterns to identify kinds of chemical reactions and to predict the products of the chemical reactions.

Types of Reactions There are five types of chemical reactions we will talk about: 1. 2. 3. 4. 5. Synthesis reactions Decomposition reactions Single replacement reactions Double replacement reactions Combustion You need to be able to identify the type of reaction

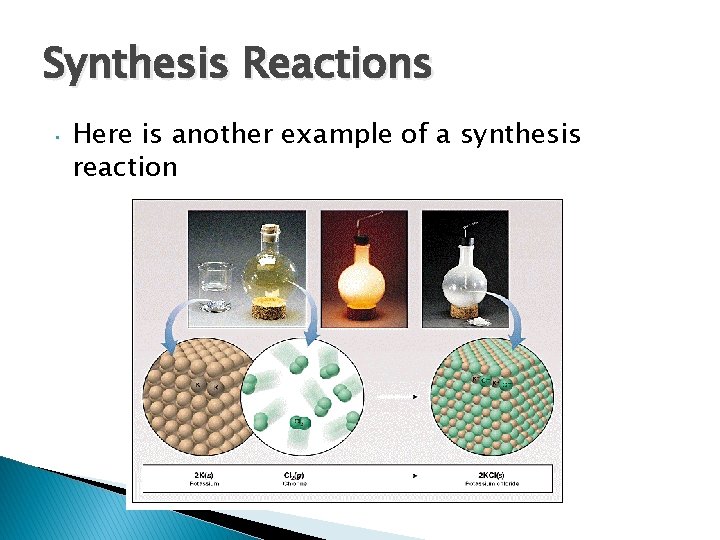
1. Synthesis Reactions Synthesis reactions occur when two substances (generally elements but sometimes compounds) combine and form a compound reactant + reactant 1 product • A + B AB • Example: 2 H 2 + O 2 2 H 2 O • Example: C + O 2 CO 2

Synthesis Reactions • Here is another example of a synthesis reaction

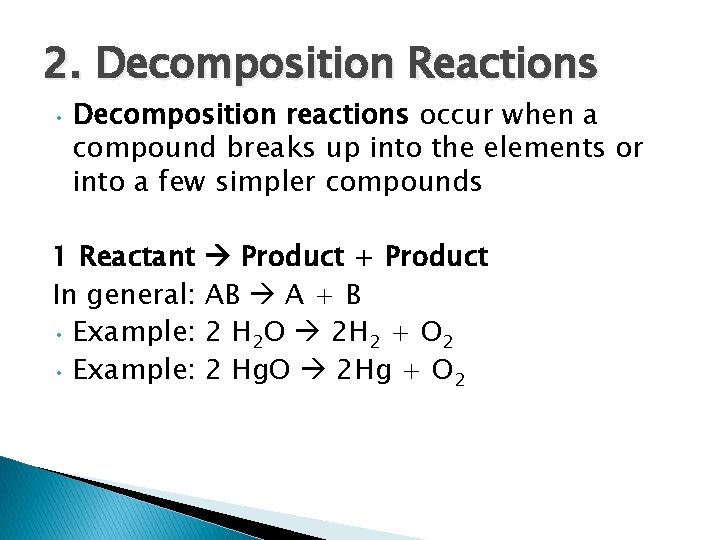
2. Decomposition Reactions • Decomposition reactions occur when a compound breaks up into the elements or into a few simpler compounds 1 Reactant Product + Product In general: AB A + B • Example: 2 H 2 O 2 H 2 + O 2 • Example: 2 Hg. O 2 Hg + O 2

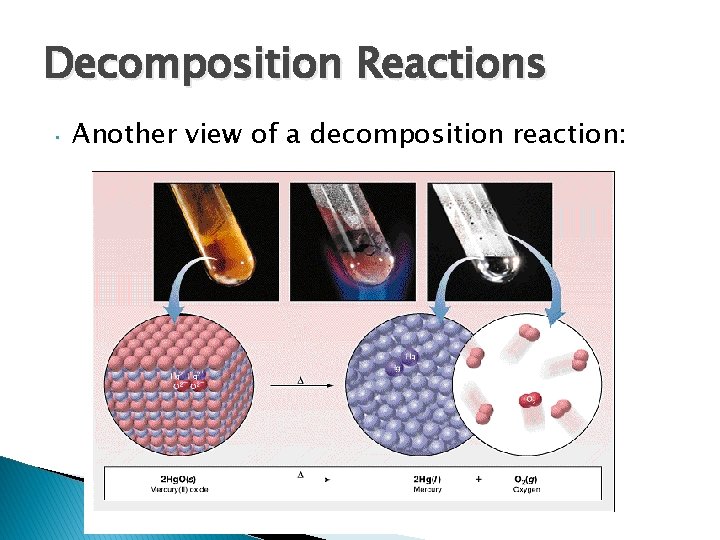
Decomposition Reactions • Another view of a decomposition reaction:

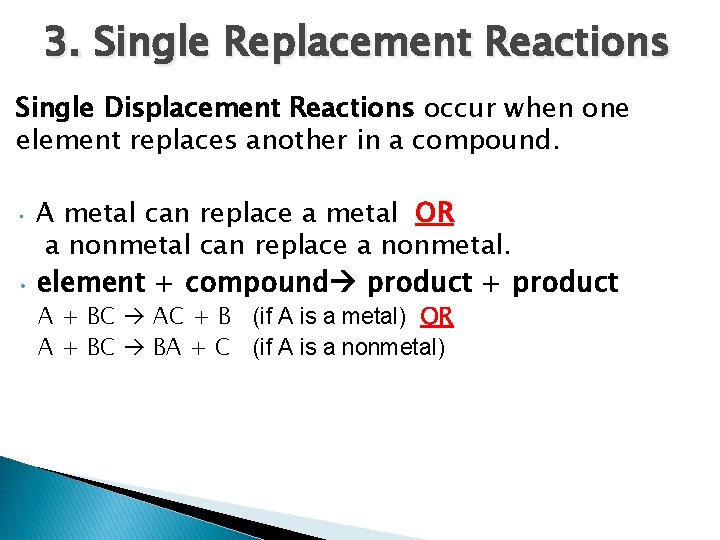
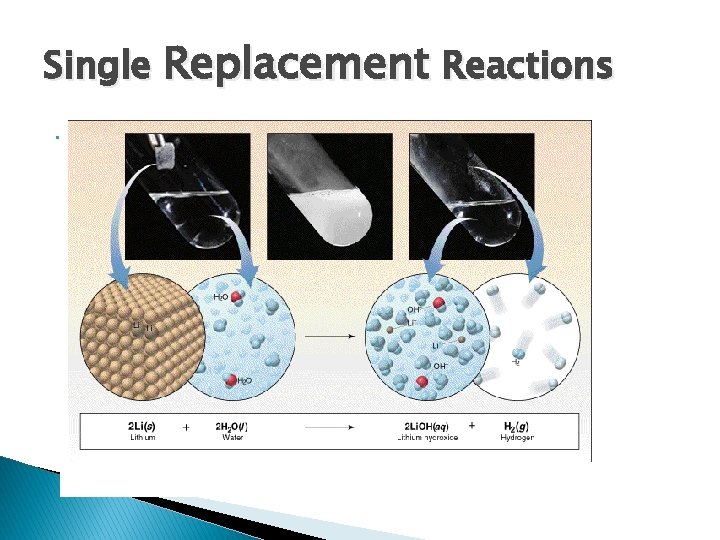
3. Single Replacement Reactions Single Displacement Reactions occur when one element replaces another in a compound. A metal can replace a metal OR a nonmetal can replace a nonmetal. • element + compound product + product • A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal)

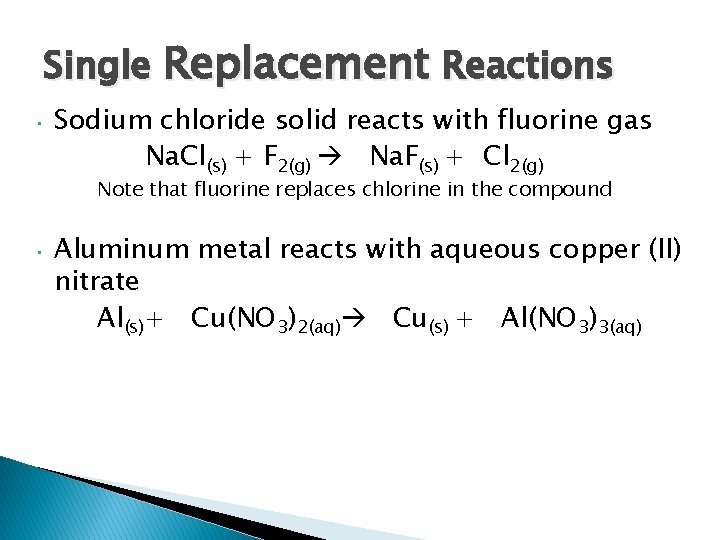
Single Replacement Reactions • Sodium chloride solid reacts with fluorine gas Na. Cl(s) + F 2(g) Na. F(s) + Cl 2(g) Note that fluorine replaces chlorine in the compound • Aluminum metal reacts with aqueous copper (II) nitrate Al(s)+ Cu(NO 3)2(aq) Cu(s) + Al(NO 3)3(aq)

Single Replacement Reactions • Another view:

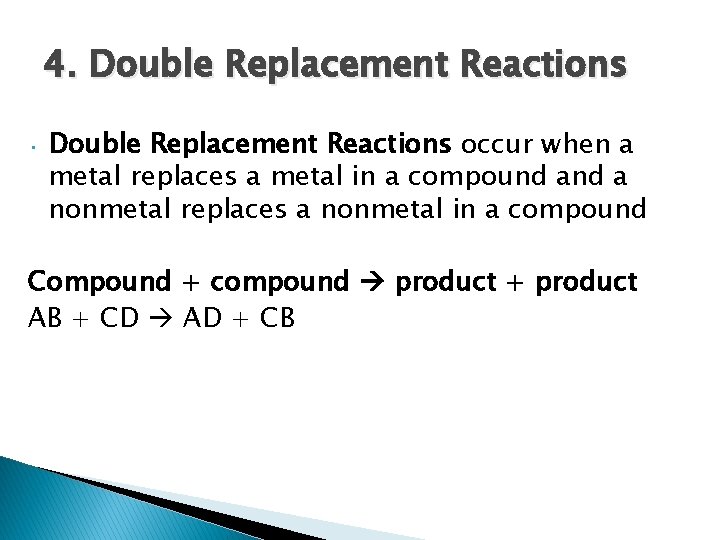
4. Double Replacement Reactions • Double Replacement Reactions occur when a metal replaces a metal in a compound a nonmetal replaces a nonmetal in a compound Compound + compound product + product AB + CD AD + CB

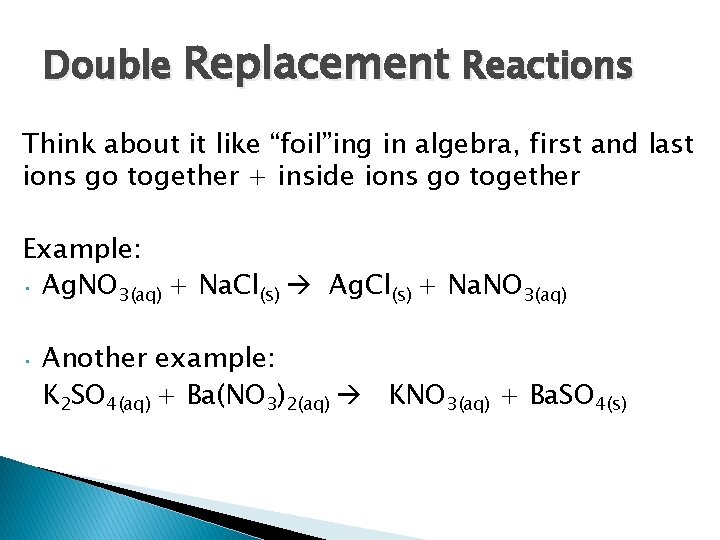
Double Replacement Reactions Think about it like “foil”ing in algebra, first and last ions go together + inside ions go together Example: • Ag. NO 3(aq) + Na. Cl(s) Ag. Cl(s) + Na. NO 3(aq) • Another example: K 2 SO 4(aq) + Ba(NO 3)2(aq) KNO 3(aq) + Ba. SO 4(s)

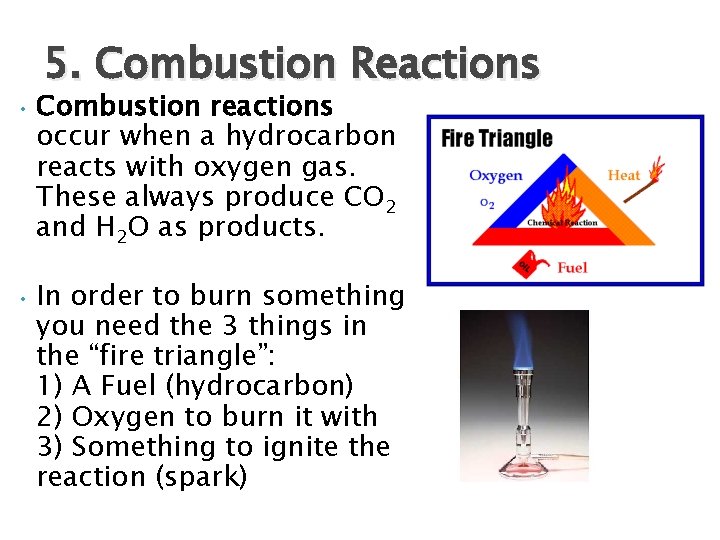
5. Combustion Reactions • Combustion reactions occur when a hydrocarbon reacts with oxygen gas. These always produce CO 2 and H 2 O as products. • In order to burn something you need the 3 things in the “fire triangle”: 1) A Fuel (hydrocarbon) 2) Oxygen to burn it with 3) Something to ignite the reaction (spark)

Combustion • Examples • C 5 H 12 +8 O 2 5 CO 2 + 6 H 2 O and