Reaction rates The speed of chemistry Reaction rates




- Slides: 13

Reaction rates The speed of chemistry

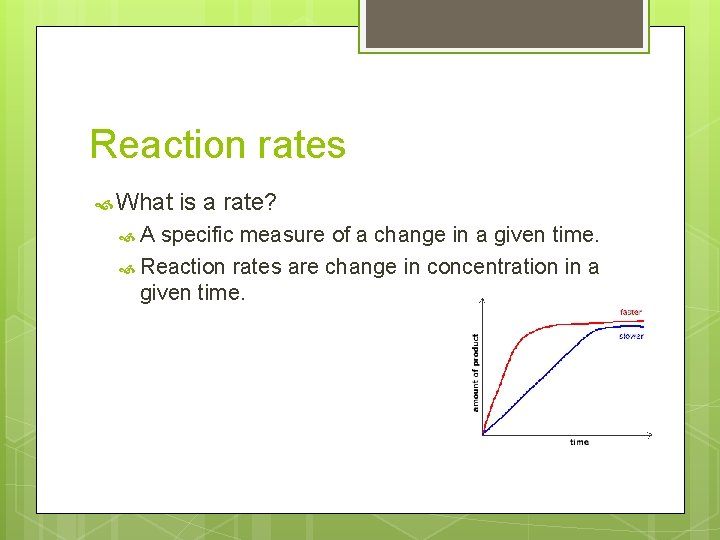
Reaction rates What A is a rate? specific measure of a change in a given time. Reaction rates are change in concentration in a given time.

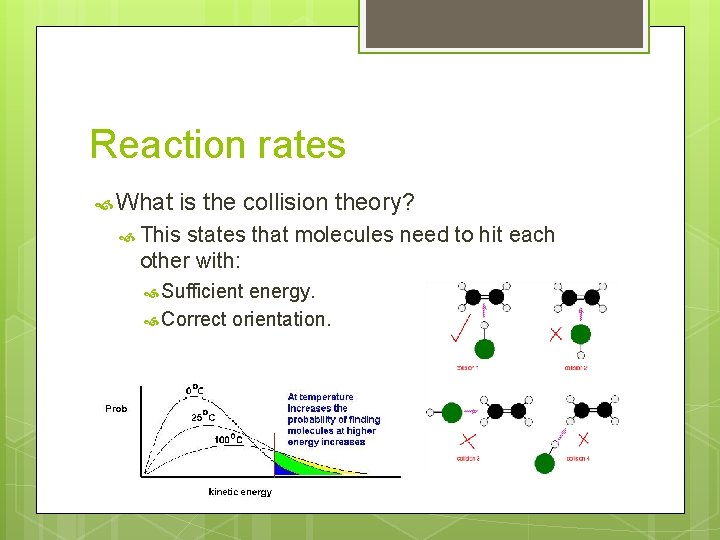
Reaction rates What is the collision theory? This states that molecules need to hit each other with: Sufficient energy. Correct orientation.

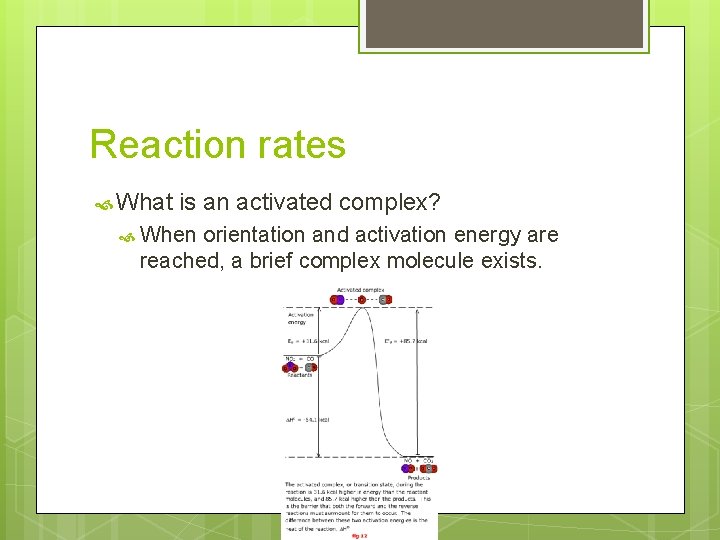
Reaction rates What is an activated complex? When orientation and activation energy are reached, a brief complex molecule exists.

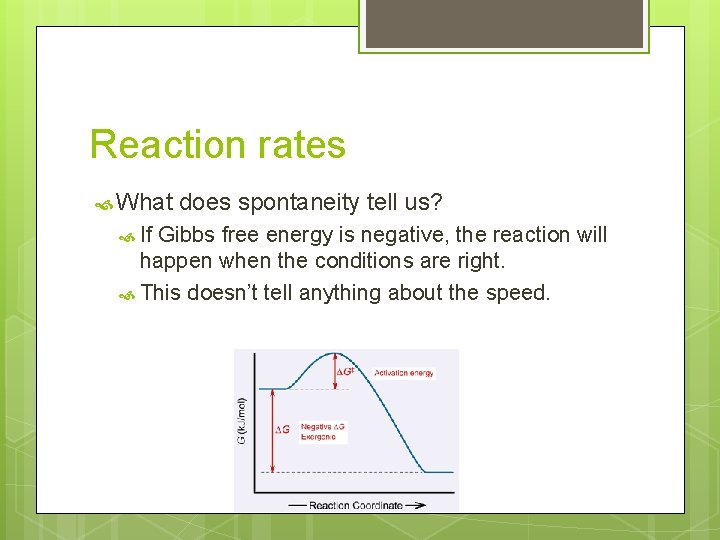
Reaction rates What If does spontaneity tell us? Gibbs free energy is negative, the reaction will happen when the conditions are right. This doesn’t tell anything about the speed.

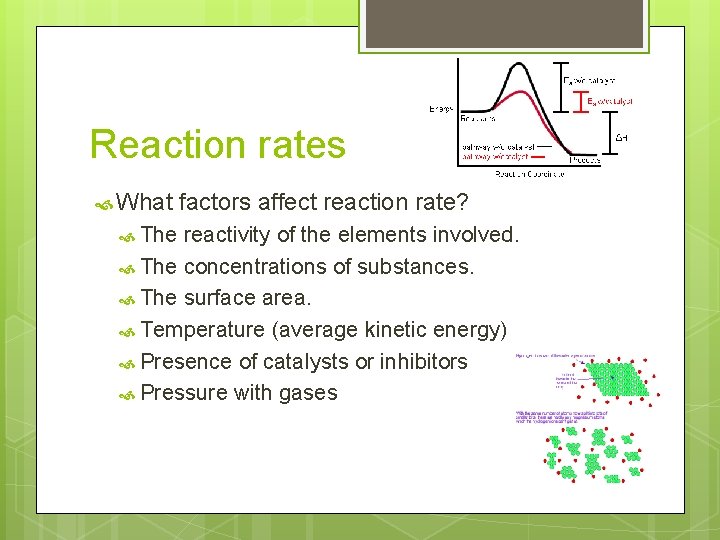
Reaction rates What The factors affect reaction rate? reactivity of the elements involved. The concentrations of substances. The surface area. Temperature (average kinetic energy) Presence of catalysts or inhibitors Pressure with gases

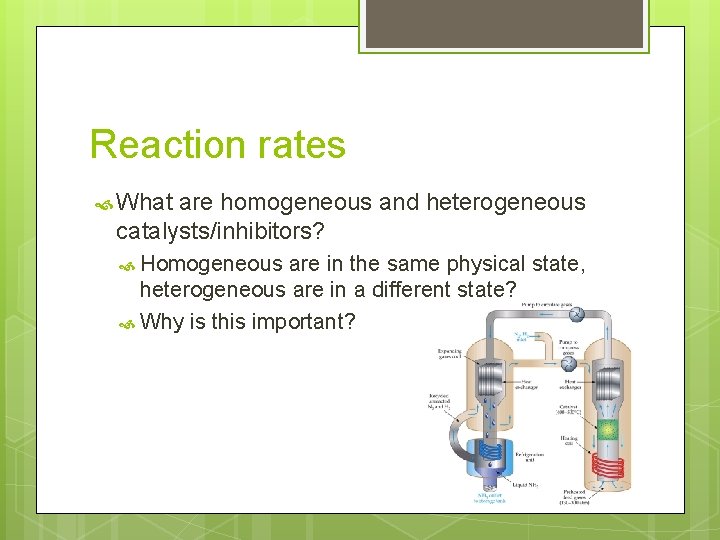
Reaction rates What are homogeneous and heterogeneous catalysts/inhibitors? Homogeneous are in the same physical state, heterogeneous are in a different state? Why is this important?

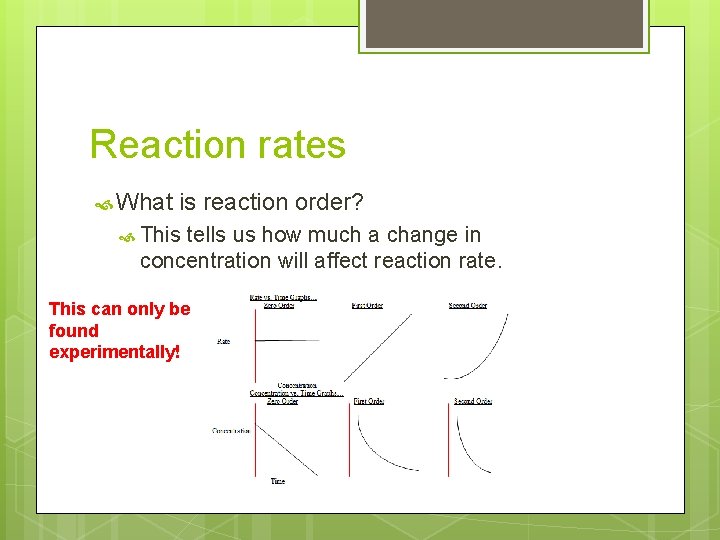
Reaction rates What is reaction order? This tells us how much a change in concentration will affect reaction rate. This can only be found experimentally!

Reaction rates What are reaction rate laws? These equations can determine rate by factoring in a rate constant, concentration and reaction order. rate=k[A] k is a constant (given) [ ] is concentration rate=k[A]m[B]n m and n are reaction orders (found or given)

Reaction rates How 0 can we find reaction order from data? order has no effect when concentration is changed. 1 st order has a doubling effect on rate when concentration is doubled. 2 nd order has a 4 x effect when doubled.

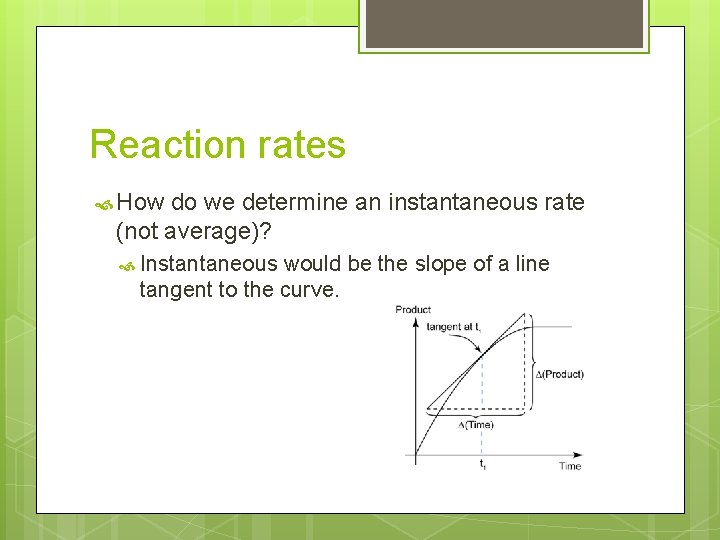
Reaction rates How do we determine an instantaneous rate (not average)? Instantaneous would be the slope of a line tangent to the curve.

Reaction rates What A is a complex reaction mechanism? reaction that doesn’t happen in one step is said to be complex. The reaction mechanism shows the intermediate steps and substances used in overall reaction. The rate-determining step is the slowest elementary step in the complex reaction.

Reaction rates