Reaction Rates Review u A chemical equation describes

Reaction Rates
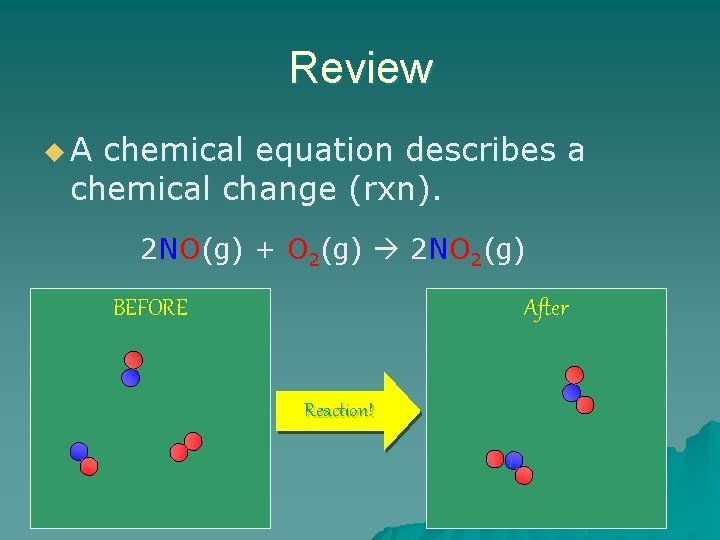
Review u. A chemical equation describes a chemical change (rxn). 2 NO(g) + O 2(g) 2 NO 2(g) BEFORE After Reaction!

Reaction Rates u Reaction rate = “speed” of reaction. – EX: Burning of hydrogen fast – EX: Rusting of iron slow – EX: Conversion of graphite to diamond extremely slow u Collision theory: reactions happen when molecules collide. – Reaction rate depends on two things (that we’re going to discuss today): u How frequently molecules collide. u How fast they are moving when they do collide.
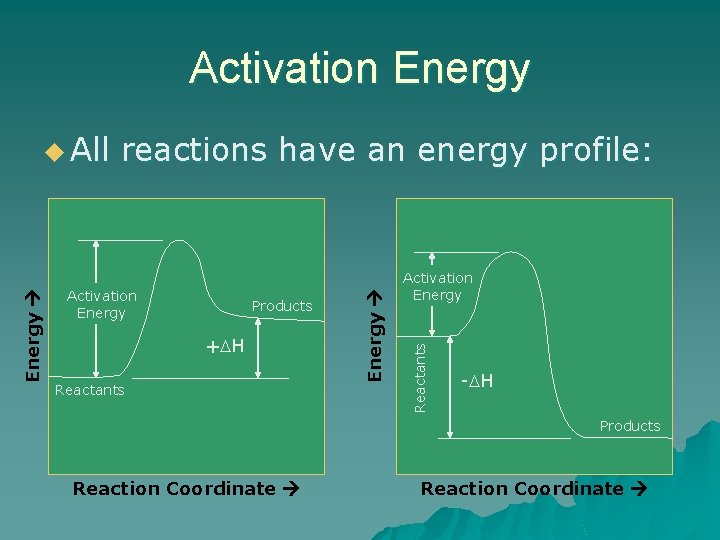
Activation Energy reactions have an energy profile: Products + H Reactants Activation Energy u All - H Products Reaction Coordinate

Collision Theory u When reactant molecules collide, they must collide with at least as much total energy as the activation energy. – Any molecules that collide with less than the activation energy will simply bounce apart w/o reacting. u The more molecules there are colliding with sufficient energy to react, the faster the reaction runs.

Temperature u In general, rxns run faster at high temperatures. – Rule of thumb: A rxn’s rate approx. doubles when the temp. rises by 10ºC. – Not always true, but generally accurate. u Rise in temp. means molecules move faster. – More collisions per second. – Collisions w/ greater energy.
Concentration u As solute concentration increases, rxn rate usually increases also. – More molecules colliding per unit of time. – EX: Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) – 6. 00 M HCl soln. will “eat” Zn much faster than 1. 00 M HCl soln. u Explains why we usually mix reactants with one reactant in great excess. – Keeps rxn rate high for entire rxn.

Concentration Mg + H 2 SO 4 Mg. SO 4 + H 2 Set-Up 1 Set-Up 2 Add 1 mole of Mg to 1 mole of H 2 SO 4. Add 1 mole of Mg to 5 moles of H 2 SO 4. Result Mg and H 2 SO 4 are both completely used up, but the reaction gets very slow close to the end. 4 moles of H 2 SO 4 remain, but the reaction stays fast all the way through.
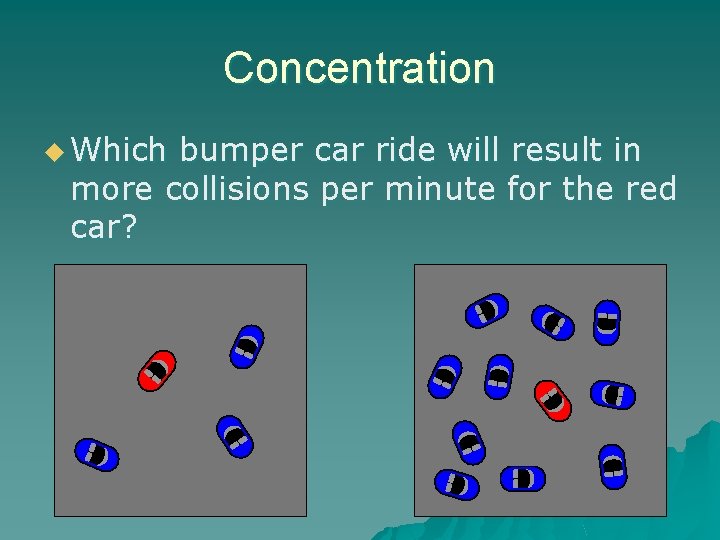
Concentration u Which bumper car ride will result in more collisions per minute for the red car?

Particle Size u The smaller the reactant particles, the faster the rxn. – Grind up solids. – Dissolve solids. u Breaking up reactant particles increases: – surface area.

Particle Size u Aluminum powder bears a warning that it is an explosion hazard. Aluminum foil bears no such warning. Why is Al powder so dangerous when Al foil is not? – Powdered Al has a much greater surface area exposed to oxygen. – The oxidation of aluminum is highly exothermic. – With powdered aluminum, a highly exothermic rxn can occur at a much greater rate.
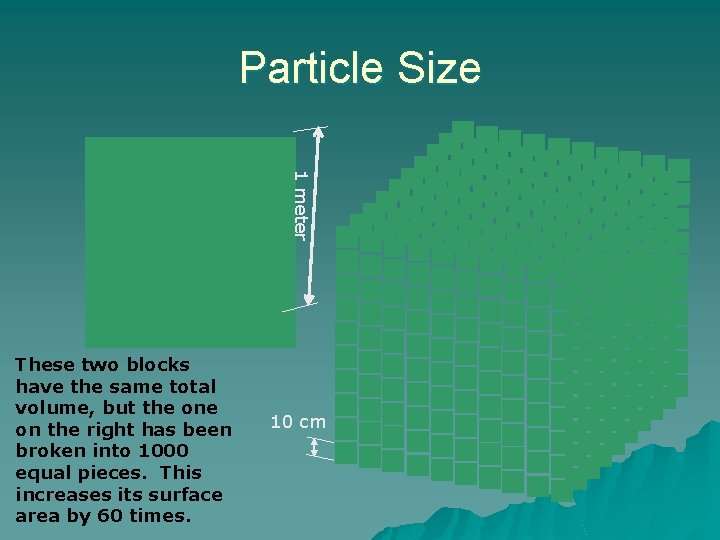
Particle Size 1 meter These two blocks have the same total volume, but the on the right has been broken into 1000 equal pieces. This increases its surface area by 60 times. 10 cm
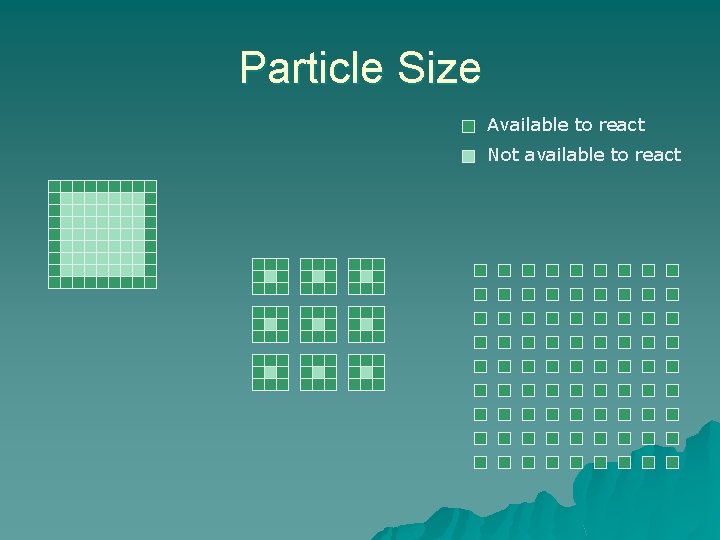
Particle Size Available to react Not available to react
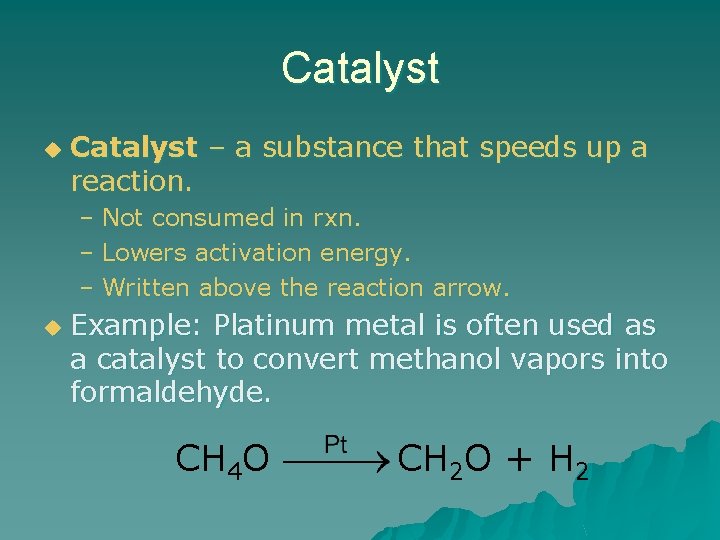
Catalyst u Catalyst – a substance that speeds up a reaction. – Not consumed in rxn. – Lowers activation energy. – Written above the reaction arrow. u Example: Platinum metal is often used as a catalyst to convert methanol vapors into formaldehyde. CH 4 O CH 2 O + H 2
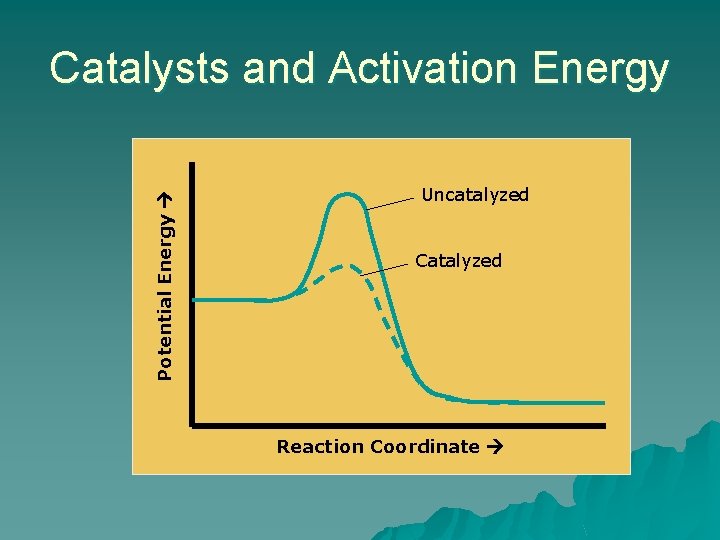
Potential Energy Catalysts and Activation Energy Uncatalyzed Catalyzed Reaction Coordinate
Catalysts u Enzymes are proteins that act as biological catalysts. – They speed up many biochemical reactions that would take far too long to happen naturally. – Enzymes are essential in just about every aspect of cell functioning. – Enzymes function best in specific temperature ranges. u Human enzymes work best around 37ºC.
- Slides: 16