Reaction rates Particle theory All matter is made













- Slides: 20

Reaction rates

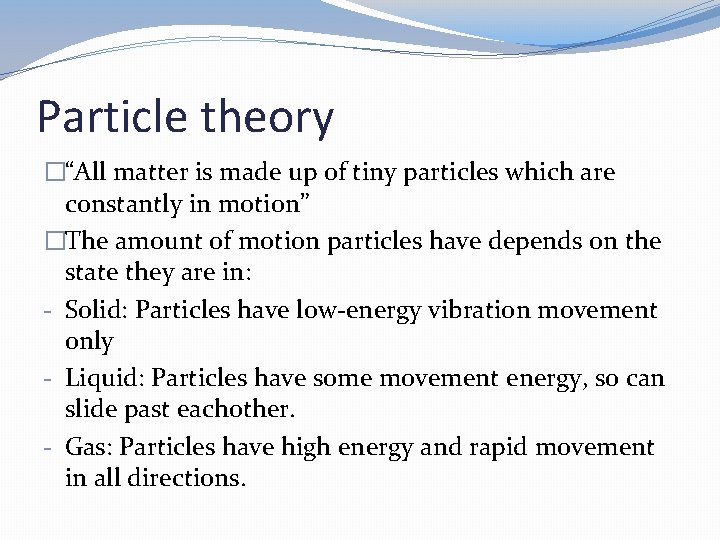
Particle theory �“All matter is made up of tiny particles which are constantly in motion”

Particle theory �“All matter is made up of tiny particles which are constantly in motion” �The amount of motion particles have depends on the state they are in:

Particle theory �“All matter is made up of tiny particles which are constantly in motion” �The amount of motion particles have depends on the state they are in: - Solid: Particles have low-energy vibration movement only

Particle theory �“All matter is made up of tiny particles which are constantly in motion” �The amount of motion particles have depends on the state they are in: - Solid: Particles have low-energy vibration movement only - Liquid: Particles have some movement energy, so can slide past eachother.

Particle theory �“All matter is made up of tiny particles which are constantly in motion” �The amount of motion particles have depends on the state they are in: - Solid: Particles have low-energy vibration movement only - Liquid: Particles have some movement energy, so can slide past eachother. - Gas: Particles have high energy and rapid movement in all directions.

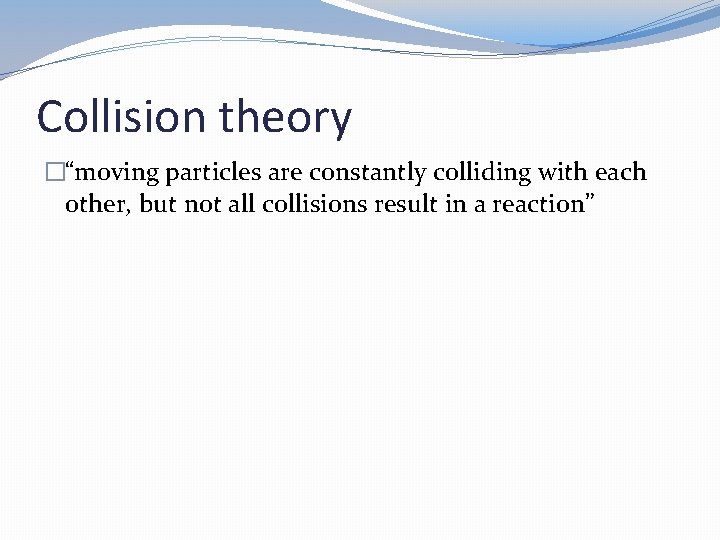
Collision theory �“moving particles are constantly colliding with each other, but not all collisions result in a reaction”

Collision theory �“moving particles are constantly colliding with each other, but not all collisions result in a reaction” �For a successful collision to occur:

Collision theory �“moving particles are constantly colliding with each other, but not all collisions result in a reaction” �For a successful collision to occur: - There must be a collision between the reactant particles.

Collision theory �“moving particles are constantly colliding with each other, but not all collisions result in a reaction” �For a successful collision to occur: - There must be a collision between the reactant particles. - The particles must collide with sufficient energy to break forces holding particles together.

Collision theory �“moving particles are constantly colliding with each other, but not all collisions result in a reaction” �For a successful collision to occur: - There must be a collision between the reactant particles. - The particles must collide with sufficient energy to break forces holding particles together. - The particles must collide in the correct position.

Collision theory �“moving particles are constantly colliding with each other, but not all collisions result in a reaction” �For a successful collision to occur: - There must be a collision between the reactant particles. - The particles must collide with sufficient energy to break forces holding particles together. - The particles must collide in the correct position. �If particles don’t do all these things, there will be no reaction.

Collision theory �When there are more collisions, there is a likelihood there will be more successful collisions, so the rate of reaction increases.

Now… �Add equal amounts of HCl to 2 test tubes �Add what you think are equal amount of calcium carbonate chips and powder to each test tube (not the same one) �Time each reaction

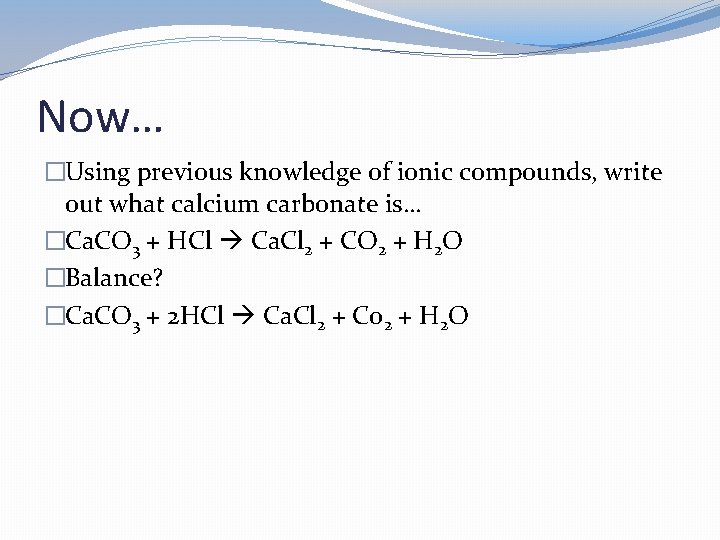
Now… �Using previous knowledge of ionic compounds, write out what calcium carbonate is…

Now… �Using previous knowledge of ionic compounds, write out what calcium carbonate is… �What is hydrochloric acid? �Write a word equation to what you think happened.

Now… �Using previous knowledge of ionic compounds, write out what calcium carbonate is… �What is hydrochloric acid? �Write a word equation to what you think happened. �Calcium carbonate + Hydrochloric acid calcium chloride + carbon dioxide + water

Now… �Using previous knowledge of ionic compounds, write out what calcium carbonate is… �Ca. CO 3 + HCl Ca. Cl 2 + CO 2 + H 2 O

Now… �Using previous knowledge of ionic compounds, write out what calcium carbonate is… �Ca. CO 3 + HCl Ca. Cl 2 + CO 2 + H 2 O �Balance?

Now… �Using previous knowledge of ionic compounds, write out what calcium carbonate is… �Ca. CO 3 + HCl Ca. Cl 2 + CO 2 + H 2 O �Balance? �Ca. CO 3 + 2 HCl Ca. Cl 2 + Co 2 + H 2 O