Reaction Rates Exothermic and Endothermic Reactions An exothermic



- Slides: 10

Reaction Rates

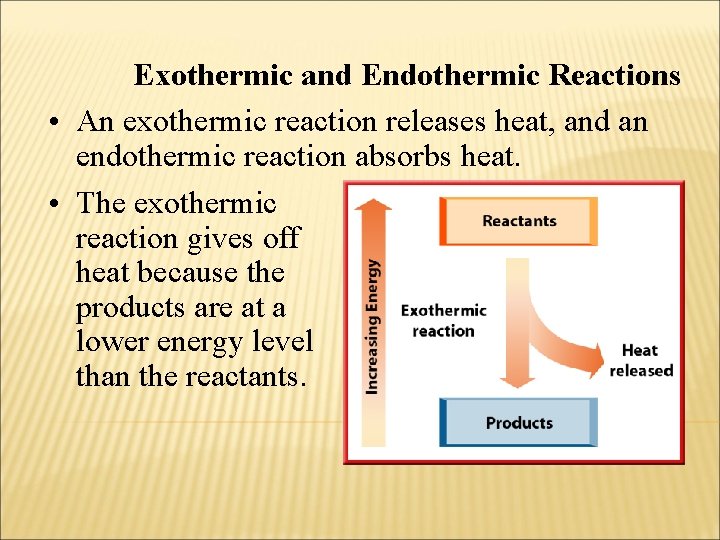
Exothermic and Endothermic Reactions • An exothermic reaction releases heat, and an endothermic reaction absorbs heat. • The exothermic reaction gives off heat because the products are at a lower energy level than the reactants.

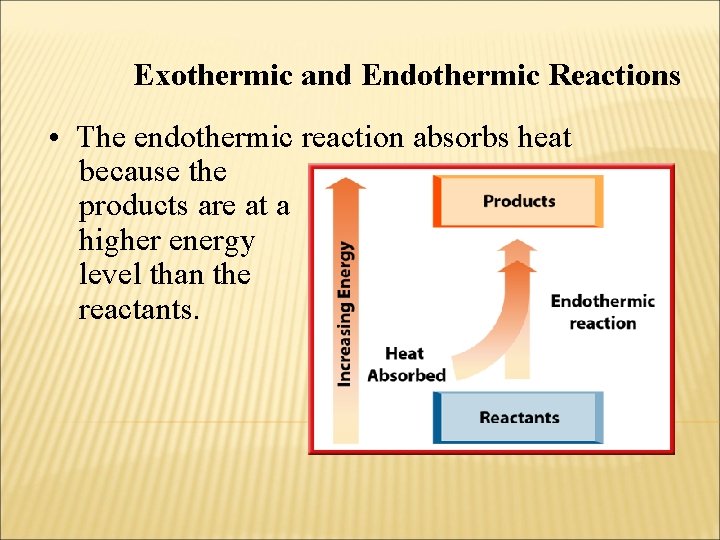
Exothermic and Endothermic Reactions • The endothermic reaction absorbs heat because the products are at a higher energy level than the reactants.

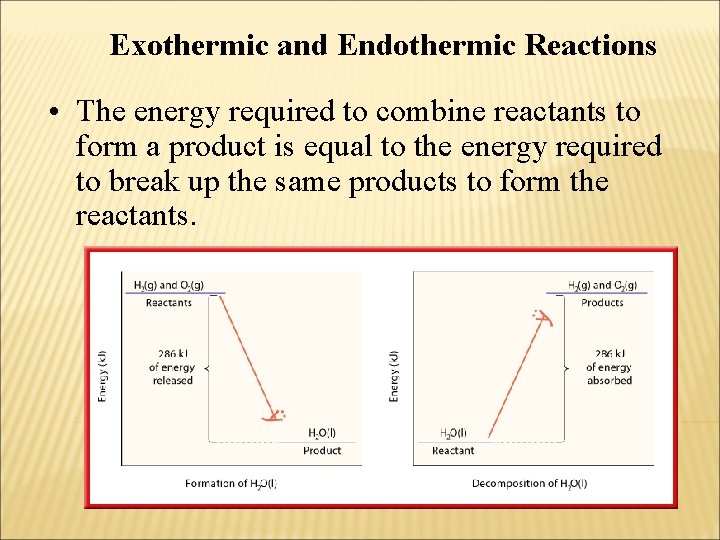
Exothermic and Endothermic Reactions • The energy required to combine reactants to form a product is equal to the energy required to break up the same products to form the reactants.

Expressing Reaction Rates • The rate of a reaction is calculated by dividing the change in quantity of the reactants by the change in time. . • Delta (∆) before a quantity indicates a change.

Expressing Reaction Rates • The reaction rate of a chemical reaction is stated as the change in concentration of a reactant or product per unit time, expressed as mol/(L∙s). • Brackets around the formula for a substance indicate the molar concentration. For example, [NO 2] represents the molar concentration of NO 2. • Reaction rates are determined in a laboratory setting, they can’t be calculated as stoichiometric amounts can.

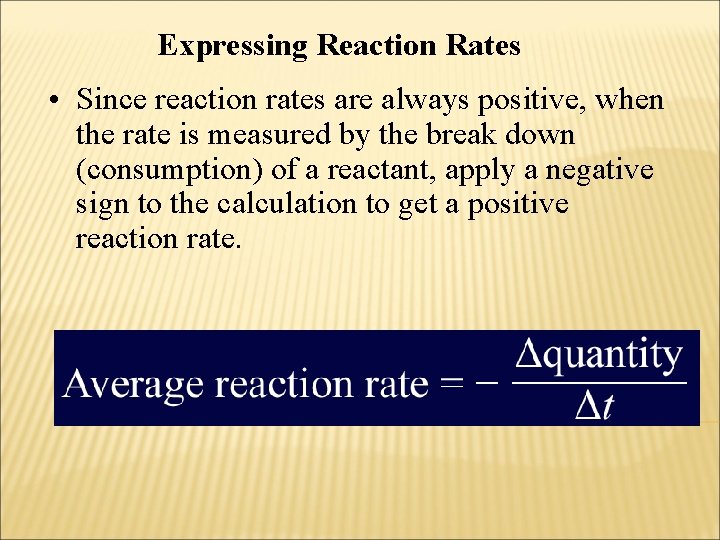
Expressing Reaction Rates • Since reaction rates are always positive, when the rate is measured by the break down (consumption) of a reactant, apply a negative sign to the calculation to get a positive reaction rate.

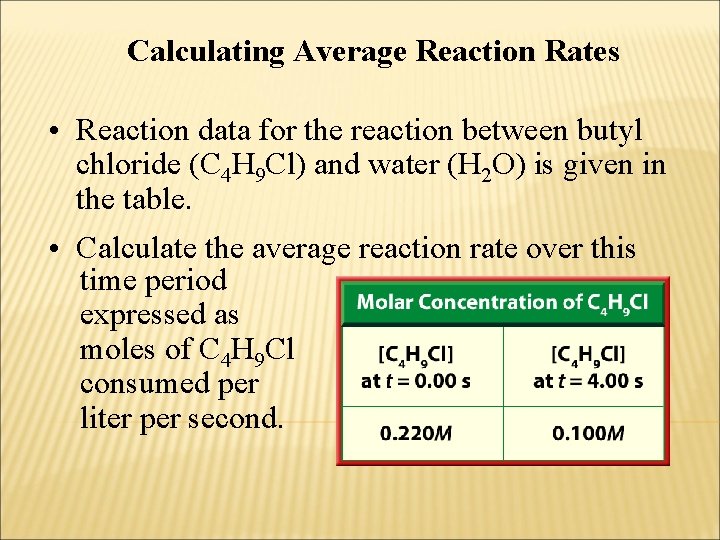
Calculating Average Reaction Rates • Reaction data for the reaction between butyl chloride (C 4 H 9 Cl) and water (H 2 O) is given in the table. • Calculate the average reaction rate over this time period expressed as moles of C 4 H 9 Cl consumed per liter per second.

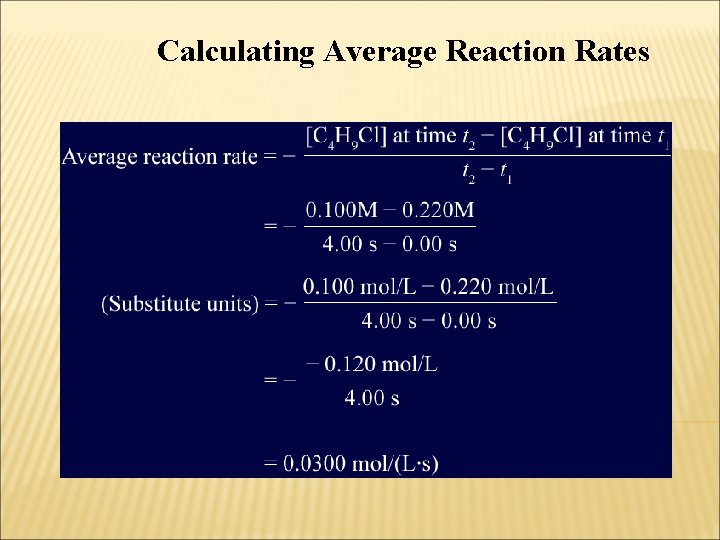
Calculating Average Reaction Rates • Write the equation for the average reaction rate, insert the known quantities, and perform the calculation.

Calculating Average Reaction Rates