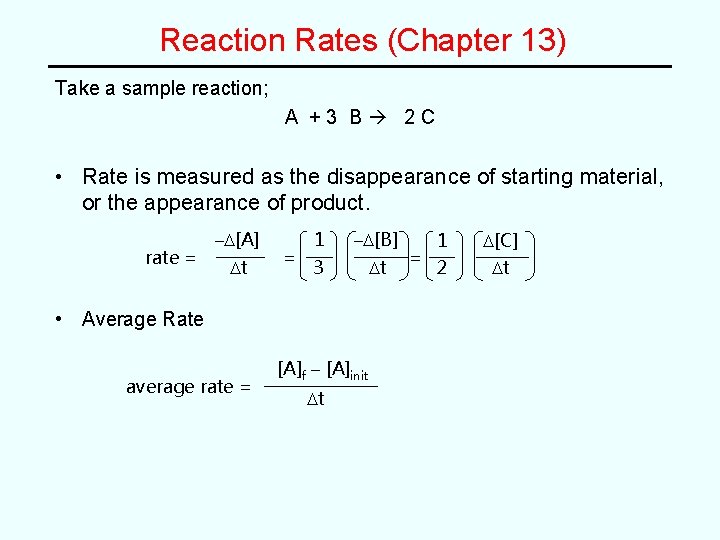
Reaction Rates Chapter 13 Take a sample reaction
![Solution (part a) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010 Solution (part a) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010](https://slidetodoc.com/presentation_image_h/2388319f25add7583881a17f6f40ab9e/image-9.jpg)
![Solution (cont) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010 7. Solution (cont) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010 7.](https://slidetodoc.com/presentation_image_h/2388319f25add7583881a17f6f40ab9e/image-10.jpg)
![Solution (part b) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010 Solution (part b) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010](https://slidetodoc.com/presentation_image_h/2388319f25add7583881a17f6f40ab9e/image-11.jpg)
- Slides: 32

Reaction Rates (Chapter 13) Take a sample reaction; A +3 B 2 C • Rate is measured as the disappearance of starting material, or the appearance of product. rate = –D[A] Dt = 1 –D[B] 3 Dt • Average Rate average rate = [A]f – [A]init Dt = 1 D[C] 2 Dt

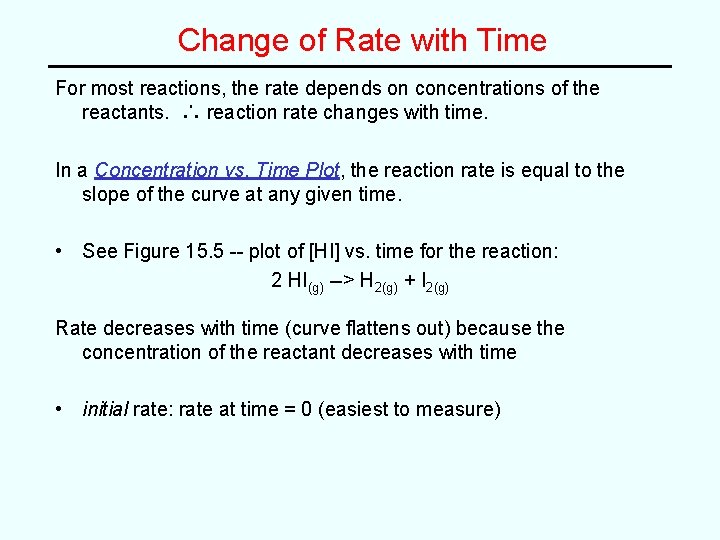
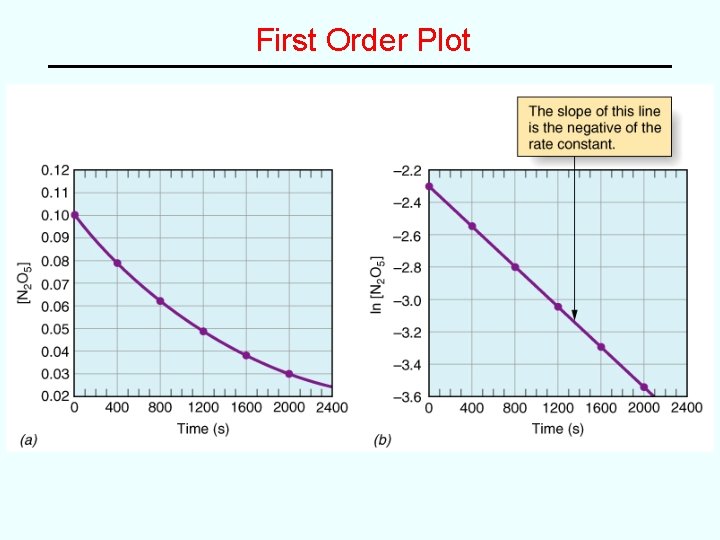
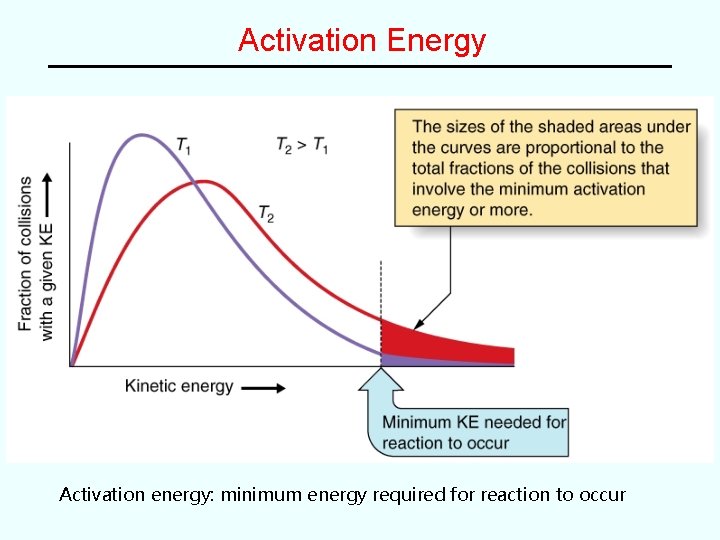
Change of Rate with Time For most reactions, the rate depends on concentrations of the reactants. ∴ reaction rate changes with time. In a Concentration vs. Time Plot, the reaction rate is equal to the slope of the curve at any given time. • See Figure 15. 5 -- plot of [HI] vs. time for the reaction: 2 HI(g) --> H 2(g) + I 2(g) Rate decreases with time (curve flattens out) because the concentration of the reactant decreases with time • initial rate: rate at time = 0 (easiest to measure)

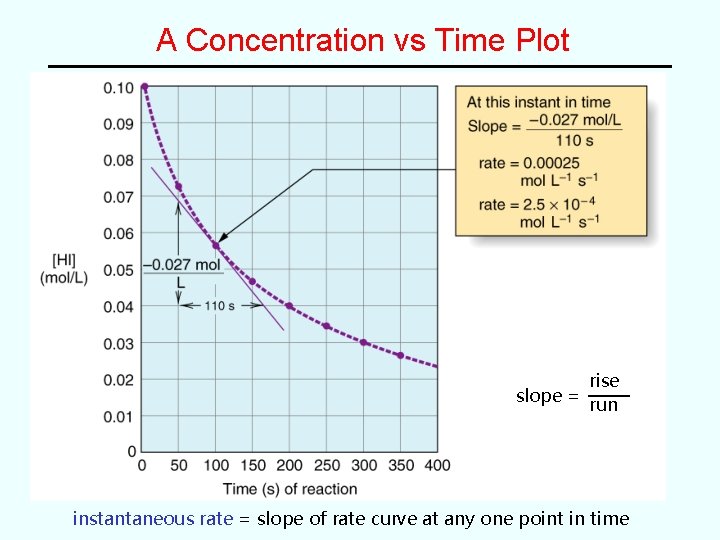
A Concentration vs Time Plot rise slope = run instantaneous rate = slope of rate curve at any one point in time

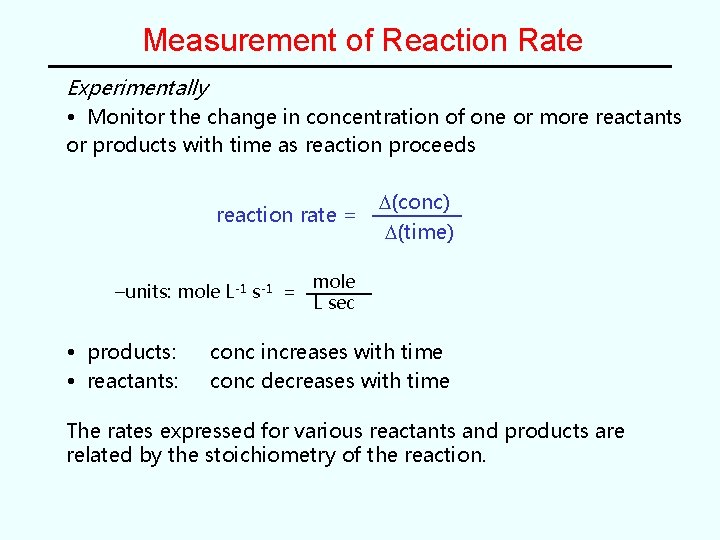
Measurement of Reaction Rate Experimentally • Monitor the change in concentration of one or more reactants or products with time as reaction proceeds reaction rate = –units: mole L-1 s-1 = • products: • reactants: D(conc) D(time) mole L sec conc increases with time conc decreases with time The rates expressed for various reactants and products are related by the stoichiometry of the reaction.

Example Problem • At a certain temperature, oxygen (O 2) is produced by the following reaction at a rate of 0. 30 mole/L sec. What are the rates of formation of the other products and the rate of disappearance of the reactant? 4 KNO 3(aq) --> 2 K 2 O(aq) + 2 N 2(g) + 5 O 2(g) rate (N 2) = 0. 30 mole O 2 2 mole N 2 0. 12 mole N 2 = x Ls 5 mole O 2 Ls Likewise, rate (K 2 O) = 0. 12 mole L sec -0. 30 mole O 2 4 mole KNO 3 -0. 24 mole KNO 3 x rate (KNO 3) = = Ls 5 mole O 2 Ls

Rate Law (concentration and reaction rate) For a general reaction: a A + b B --> products • The “Rate Law” is the following type of relationship between rate and concentrations of reactants. Rate = k[A]x[B]y {All values of k, x, and y must be determined experimentally!} • x and y are normally small integers (often 1, 2, or 0) x = “order of the reaction” with respect to A e. g. if x = 2, the rate depends on the square of [A] i. e. double [A], then rate increases by 4 times triple [A], then rate increases by 9 times, etc y = “order of the reaction” with respect to B • Overall “order” of reaction = x + y

Rate Laws In general, the exponents x and y are not equal to or directly related to the coefficients in the balanced equation. Rate = k[A]x[B]y where, k = “rate constant” – (units depend on x and y values) • Units: [A] and [B] are mole/L Rate is mole/L s or mole L-1 s-1

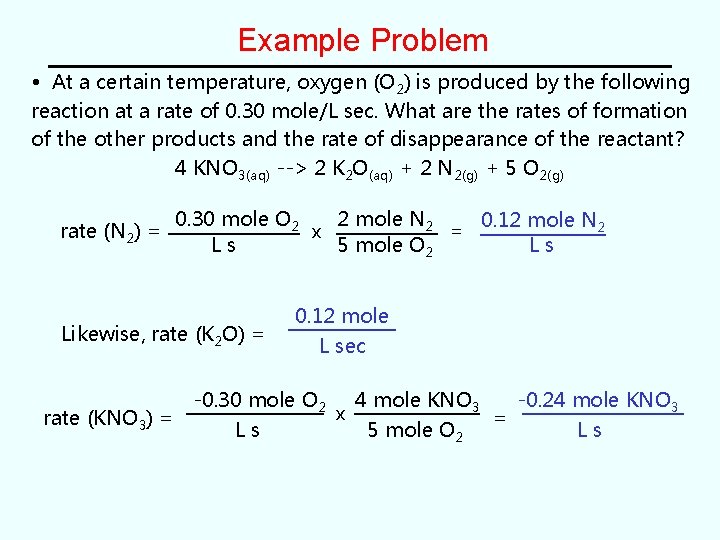
Example Problem Determination of a Rate Law from rate vs. concentration data in a kinetic study of the reaction, 2 NO(g) + O 2(g) --> 2 NO 2(g) The following rate data was obtained. Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010 7. 10 2 0. 0010 0. 0040 28. 4 3 0. 0030 0. 0040 256 a) Determine the rate law for this reaction. b) Determine the rate constant, with its proper units.
![Solution part a Expt NO O 2 Rate mole L1 s1 1 0 0010 Solution (part a) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010](https://slidetodoc.com/presentation_image_h/2388319f25add7583881a17f6f40ab9e/image-9.jpg)
Solution (part a) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010 7. 10 2 0. 0010 0. 0040 28. 4 3 0. 0030 0. 0040 256 • Part (a) Rate Law: rate = k[NO]x[O 2]y First: Compare Expts 1 and 2 with constant [NO] shows how rate changes with [O 2] rate ∝ [O 2 ]y or rate 2 = rate 1 ( [O 2]2 y [O 2]1 ) 28. 4/7. 10 = {0. 0040/0. 0010}y 4 = (4)y ∴ y = 1 (1 st order in O 2)
![Solution cont Expt NO O 2 Rate mole L1 s1 1 0 0010 7 Solution (cont) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010 7.](https://slidetodoc.com/presentation_image_h/2388319f25add7583881a17f6f40ab9e/image-10.jpg)
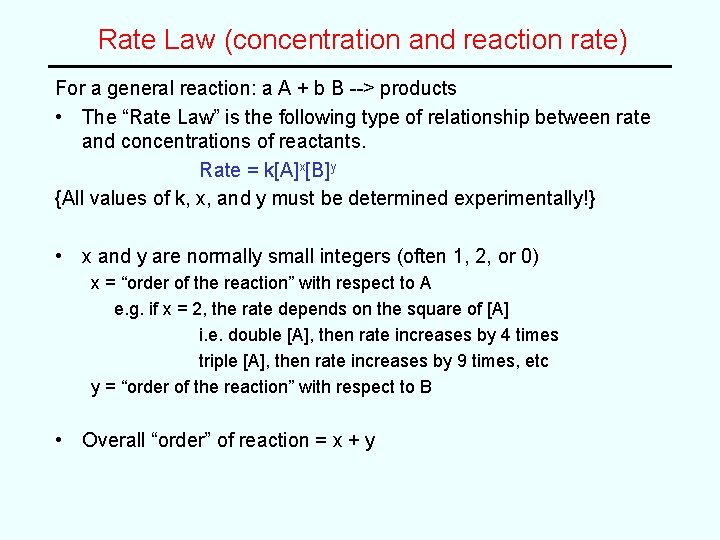
Solution (cont) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010 7. 10 2 0. 0010 0. 0040 28. 4 3 0. 0030 0. 0040 256 Second: Compare Expts 2 and 3 with constant [O 2] shows how rate changes with [NO] rate ∝ [NO]x [NO]3 rate 3 or = rate 2 [NO]2 ( x ) 256/28. 4 = {0. 0030/0. 0010}x 9. 0 = (3)x ∴ x = 2 (2 nd order in NO) • Overall Rate Law: rate = k[NO]2[O 2]
![Solution part b Expt NO O 2 Rate mole L1 s1 1 0 0010 Solution (part b) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010](https://slidetodoc.com/presentation_image_h/2388319f25add7583881a17f6f40ab9e/image-11.jpg)
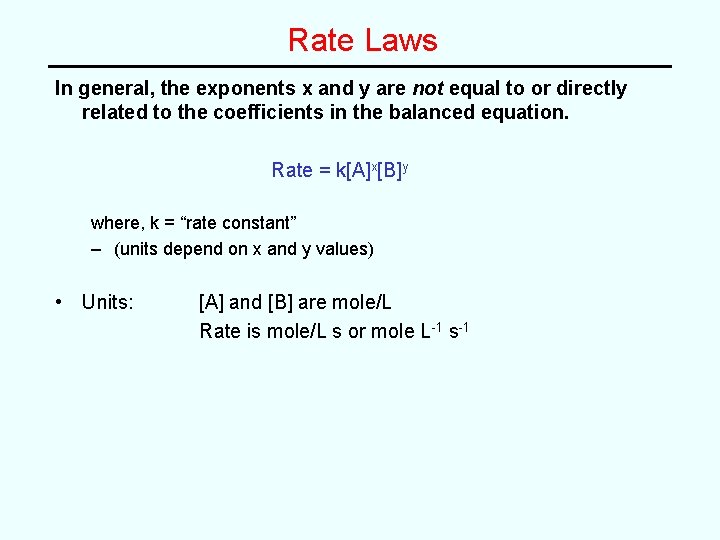
Solution (part b) Expt [NO] [O 2] Rate (mole L-1 s-1) 1 0. 0010 7. 10 2 0. 0010 0. 0040 28. 4 3 0. 0030 0. 0040 256 • Part (b) Use data from any single expt to calc k (all expts should give same value for k) e. g. from Expt 1: rate = k[NO]2[O 2] 7. 10 mole L-1 s-1 = k(0. 0010 mole L-1)2(0. 0010 mole L-1) ∴ k = 7. 1 x 109 L 2 mole-2 s-1 {Note: units of k must be determined algebraically}

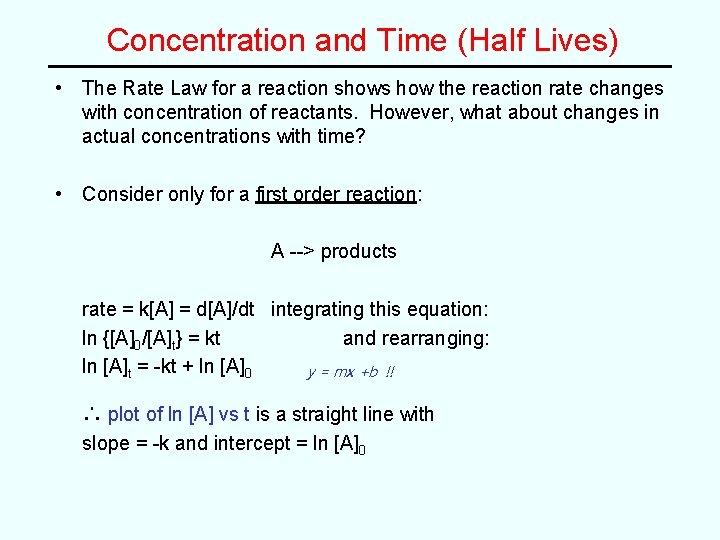
Concentration and Time (Half Lives) • The Rate Law for a reaction shows how the reaction rate changes with concentration of reactants. However, what about changes in actual concentrations with time? • Consider only for a first order reaction: A --> products rate = k[A] = d[A]/dt integrating this equation: ln {[A]0/[A]t} = kt and rearranging: ln [A]t = -kt + ln [A]0 y = mx +b !! ∴ plot of ln [A] vs t is a straight line with slope = -k and intercept = ln [A]0

First Order Plot

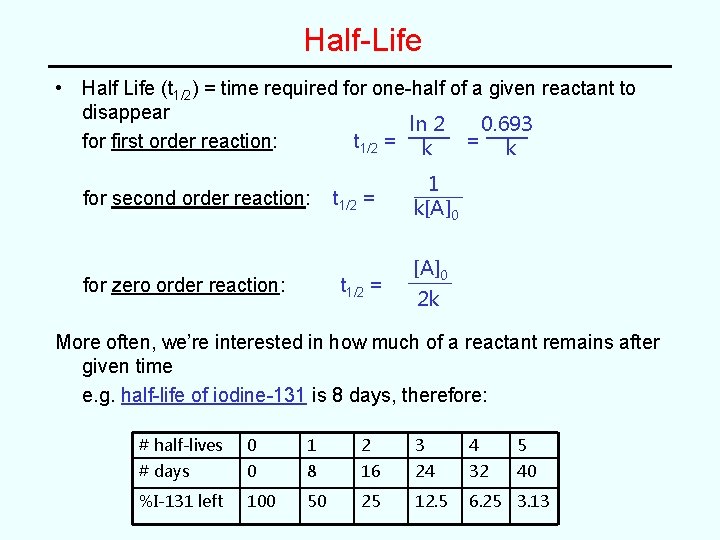
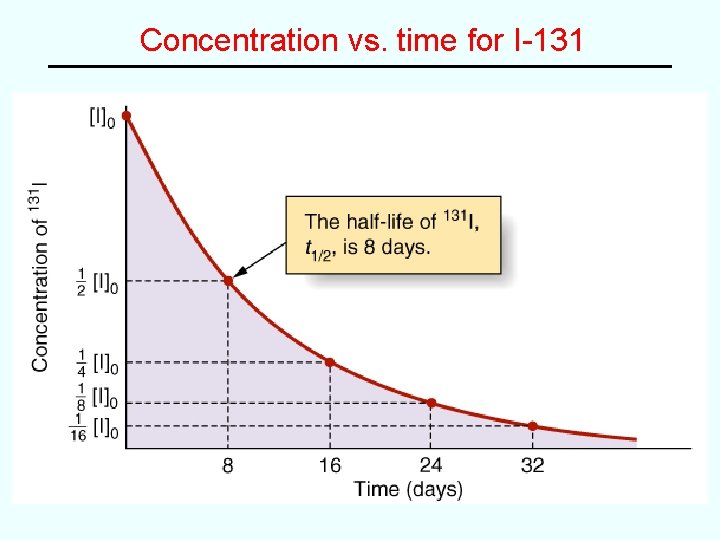
Half-Life • Half Life (t 1/2) = time required for one-half of a given reactant to disappear 0. 693 ln 2 for first order reaction: t 1/2 = k for second order reaction: for zero order reaction: t 1/2 = 1 k[A]0 2 k More often, we’re interested in how much of a reactant remains after given time e. g. half-life of iodine-131 is 8 days, therefore: # half-lives 0 1 2 3 4 5 # days 0 8 16 24 32 40 %I-131 left 100 50 25 12. 5 6. 25 3. 13

Concentration vs. time for I-131

Sample Problems 1. In the reaction, 2 A + B ⇌ 3 C, if [A] decreases at a rate of 0. 090 mol/L s, then [C] increases at a rate of _____ mole/L s. 2. The half-life of a first order reaction is 45 min. After a period of 3 hours, the percent of the original reactant present is ____%. The rate constant (k) for this same reaction is ______ s-1.

Collision Theory • Rate of a reaction depends on the frequency of effective collisions between the reactant molecules. • To be “effective” collisions must occur with proper orientation and sufficient energy. Activation Energy (Ea) = minimum energy required for reaction to occur

Activation Energy Activation energy: minimum energy required for reaction to occur

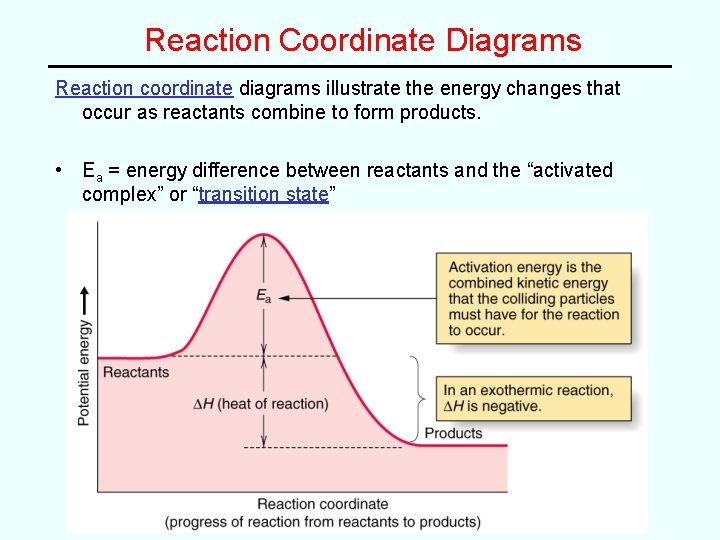
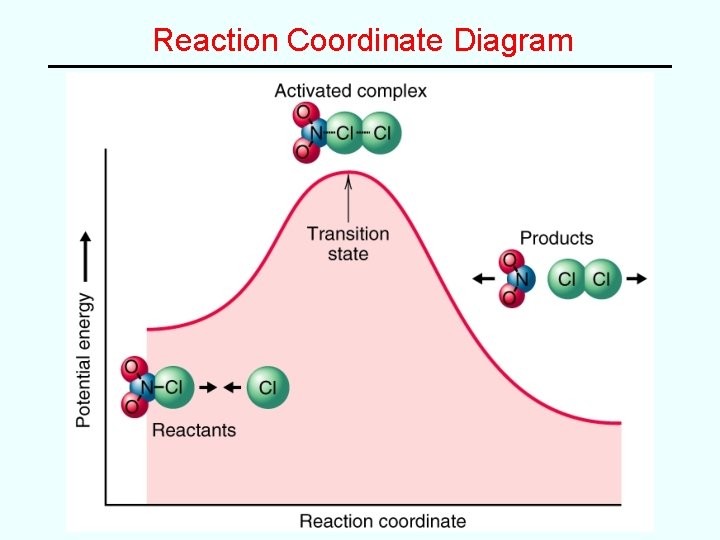
Reaction Coordinate Diagrams Reaction coordinate diagrams illustrate the energy changes that occur as reactants combine to form products. • Ea = energy difference between reactants and the “activated complex” or “transition state”

Reaction Coordinate Diagram

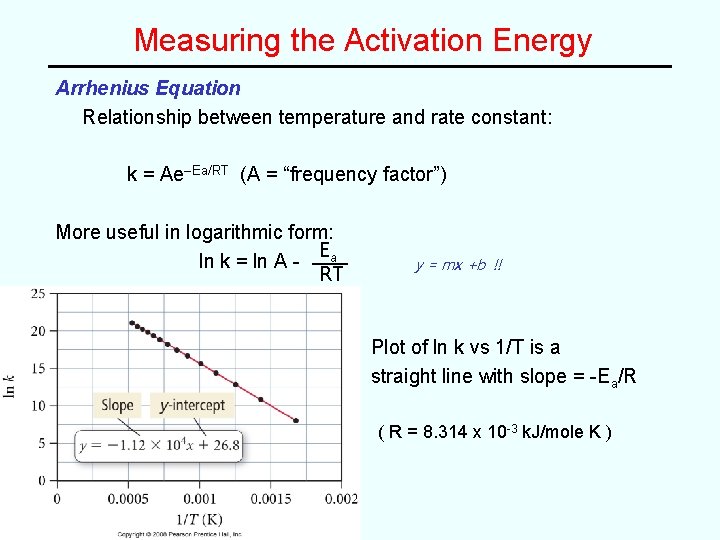
Measuring the Activation Energy Arrhenius Equation Relationship between temperature and rate constant: k = Ae–Ea/RT (A = “frequency factor”) More useful in logarithmic form: Ea ln k = ln A RT y = mx +b !! Plot of ln k vs 1/T is a straight line with slope = -Ea/R ( R = 8. 314 x 10 -3 k. J/mole K )

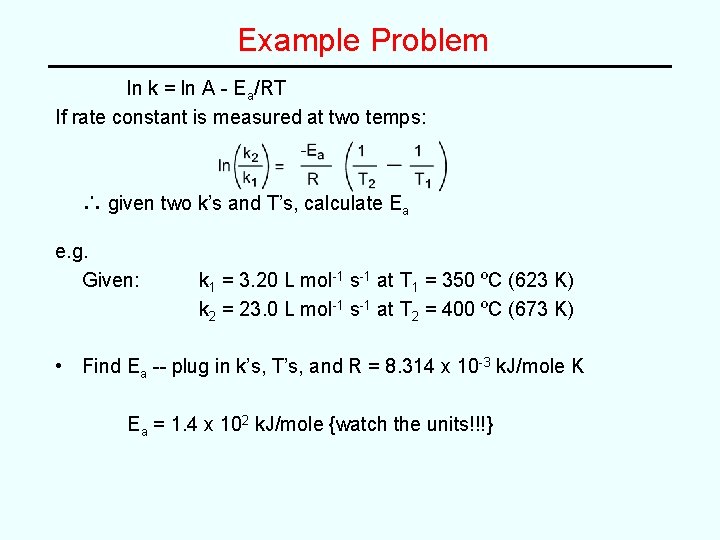
Example Problem ln k = ln A - Ea/RT If rate constant is measured at two temps: ∴ given two k’s and T’s, calculate Ea e. g. Given: k 1 = 3. 20 L mol-1 s-1 at T 1 = 350 ºC (623 K) k 2 = 23. 0 L mol-1 s-1 at T 2 = 400 ºC (673 K) • Find Ea -- plug in k’s, T’s, and R = 8. 314 x 10 -3 k. J/mole K Ea = 1. 4 x 102 k. J/mole {watch the units!!!}

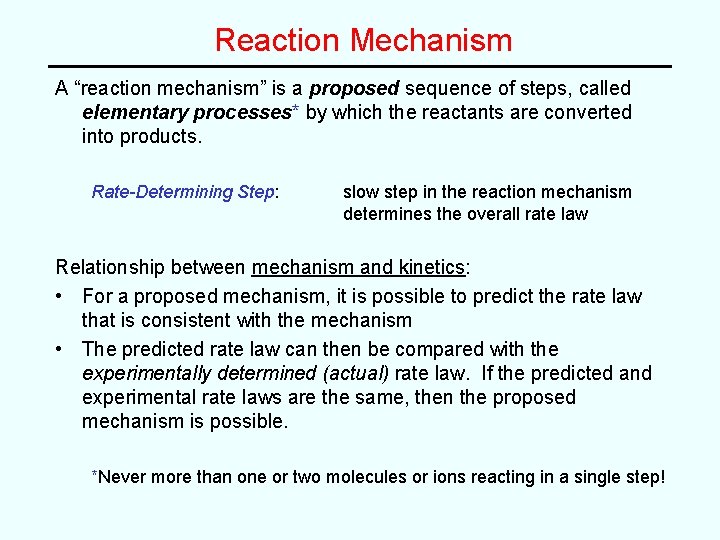
Reaction Mechanism A “reaction mechanism” is a proposed sequence of steps, called elementary processes* by which the reactants are converted into products. Rate-Determining Step: slow step in the reaction mechanism determines the overall rate law Relationship between mechanism and kinetics: • For a proposed mechanism, it is possible to predict the rate law that is consistent with the mechanism • The predicted rate law can then be compared with the experimentally determined (actual) rate law. If the predicted and experimental rate laws are the same, then the proposed mechanism is possible. *Never more than one or two molecules or ions reacting in a single step!

Predicting Rate Law from Mechanism For an elementary process, the exponents in the rate expression are equal to the coefficients in the balanced equation for that individual process. e. g. for the overall reaction, NO 2 + CO --> NO + CO 2 The following mechanism is proposed. slow: NO 2 + NO 2 --> NO + NO 3 (an “intermediate”) fast: NO 3 + CO --> NO 2 + CO 2 net: NO 2 + CO --> NO + CO 2 The slow step, predicts: rate = k[NO 2]2 The actual, experimental rate law: rate = k[NO 2]2 ∴ this is a possible mechanism! The slow step is the rate-determining step.

Determining Possible Mechanisms What about an alternate, one-step mechanism? Slow: NO 2 + CO --> NO + CO 2 • This would predict: rate = k[NO 2][CO] which is not observed experimentally. ∴ this is not a possible mechanism! What about an alternate, two-step mechanism? Fast: NO 2 + NO 2 --> NO + NO 3 Slow: NO 3 + CO --> NO 2 + CO 2 (rds) Net: NO 2 + CO --> NO + CO 2 In this case, the predicted rate law is: rate = k[NO 2]2[CO] ∴ this is not a possible mechanism!

Sample Problem A study of the kinetics of the gas-phase reaction of nickel carbonyl, Ni(CO)4, with phosphorus trifluoride, PF 3, gave the following initial rate vs concentration data. Ni(CO)4 + PF 3 --> Ni(CO)3 PF 3 + CO Expt [Ni(CO)4] [PF 3] Rate (mole/L s) (1) 0. 10 0. 25 9. 60 x 10 -4 (2) 0. 20 0. 50 1. 92 x 10 -3 (3) 0. 50 4. 80 x 10 -3 (4) 0. 50 0. 25 4. 80 x 10 -3 a) Determine the rate law for this reaction. b) Determine the value for the rate constant (k) for the above reaction.

Sample Problem, continued… Ni(CO)4 + PF 3 --> Ni(CO)3 PF 3 + CO Expt [Ni(CO)4] [PF 3] Rate (mole/L s) (1) 0. 10 0. 25 9. 60 x 10 -4 (2) 0. 20 0. 50 1. 92 x 10 -3 (3) 0. 50 4. 80 x 10 -3 (4) 0. 50 0. 25 4. 80 x 10 -3 c) Given the experimental rate law, as determined above, is it possible for this reaction to occur by a simple one-step mechanism? Why or why not? d) If the answer to c) is No, then propose a mechanism for this process that is consistent with the rate law.

Factors that Affect Reaction Rate • Nature of the Reactants: – Molecular structure, bond polarity, physical state, etc. • Ability of reactants to come in contact – heterogeneous reaction: reactants in different phases, e. g. solid/gas or solid/liquid – homogeneous reaction: all reactants in same phase, e. g. reaction in solution • Concentrations of the reactants • Temperature of the system • Presence of a catalyst – (substance that increases the reaction rate without being consumed itself)

Effect of Catalysts A catalyst is a substance that increases the rate of a reaction without itself being consumed. – Two types: homogeneous or heterogeneous Mode of action: • Catalyst alters the reaction mechanism by providing a lower energy (smaller Ea) pathway for the reaction. • Catalyst often activate (weaken) one or more bonds in the reactants and/or help to properly orient the reacting molecules (especially on surface of heterogeneous catalysts), thus promoting their reaction.

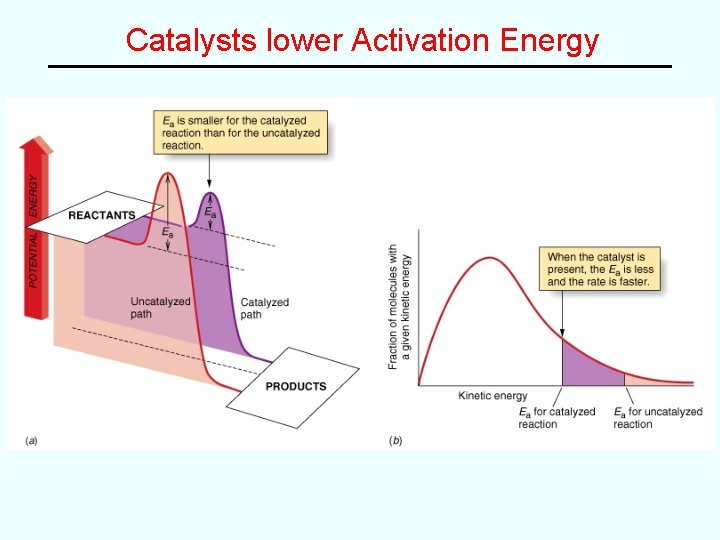
Catalysts lower Activation Energy

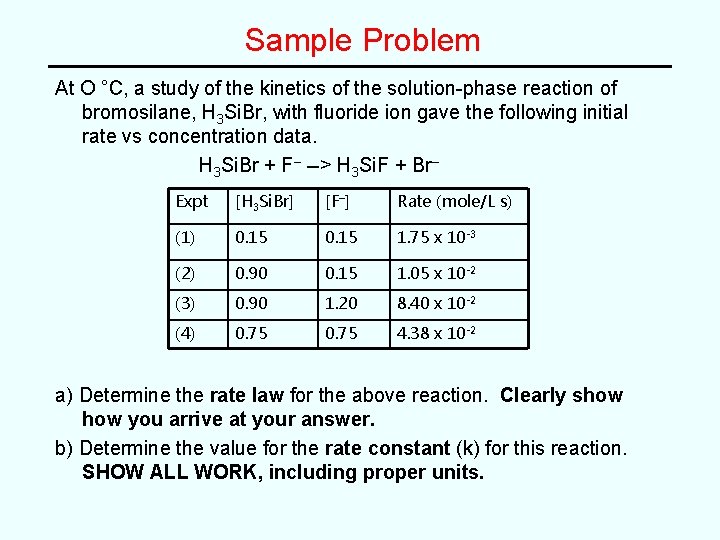
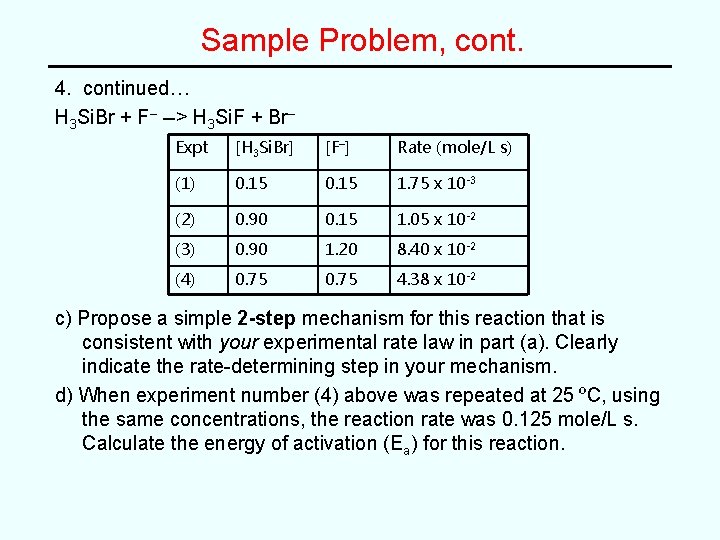
Sample Problem At O °C, a study of the kinetics of the solution-phase reaction of bromosilane, H 3 Si. Br, with fluoride ion gave the following initial rate vs concentration data. H 3 Si. Br + F– --> H 3 Si. F + Br– Expt [H 3 Si. Br] [F–] Rate (mole/L s) (1) 0. 15 1. 75 x 10 -3 (2) 0. 90 0. 15 1. 05 x 10 -2 (3) 0. 90 1. 20 8. 40 x 10 -2 (4) 0. 75 4. 38 x 10 -2 a) Determine the rate law for the above reaction. Clearly show you arrive at your answer. b) Determine the value for the rate constant (k) for this reaction. SHOW ALL WORK, including proper units.

Sample Problem, cont. 4. continued… H 3 Si. Br + F– --> H 3 Si. F + Br– Expt [H 3 Si. Br] [F–] Rate (mole/L s) (1) 0. 15 1. 75 x 10 -3 (2) 0. 90 0. 15 1. 05 x 10 -2 (3) 0. 90 1. 20 8. 40 x 10 -2 (4) 0. 75 4. 38 x 10 -2 c) Propose a simple 2 -step mechanism for this reaction that is consistent with your experimental rate law in part (a). Clearly indicate the rate-determining step in your mechanism. d) When experiment number (4) above was repeated at 25 ºC, using the same concentrations, the reaction rate was 0. 125 mole/L s. Calculate the energy of activation (Ea) for this reaction.