Reaction Rates A Reaction Rates B Collision Theory

Reaction Rates A. Reaction Rates B. Collision Theory C. 5 Factors Affecting Rates
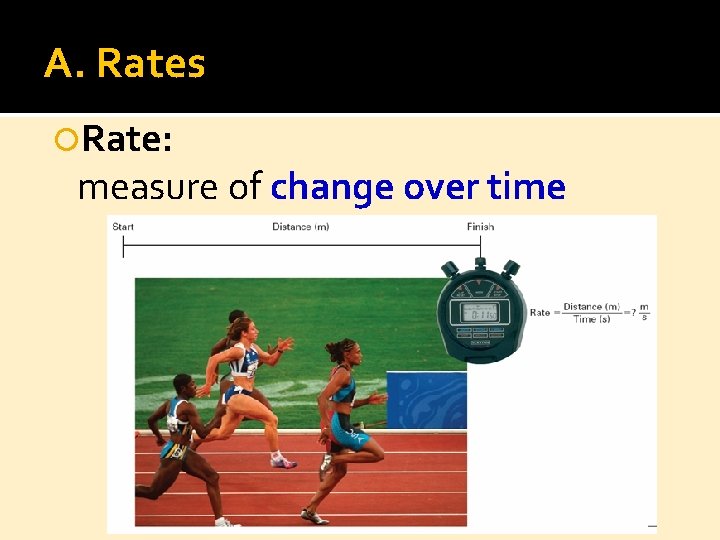
A. Rates Rate: measure of change over time
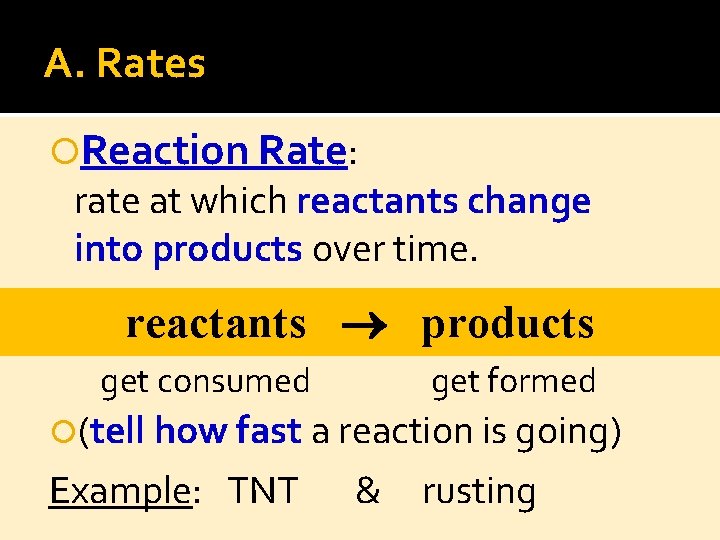
A. Rates Reaction Rate: rate at which reactants change into products over time. reactants products get formed get consumed (tell how fast a reaction is going) Example: TNT & rusting
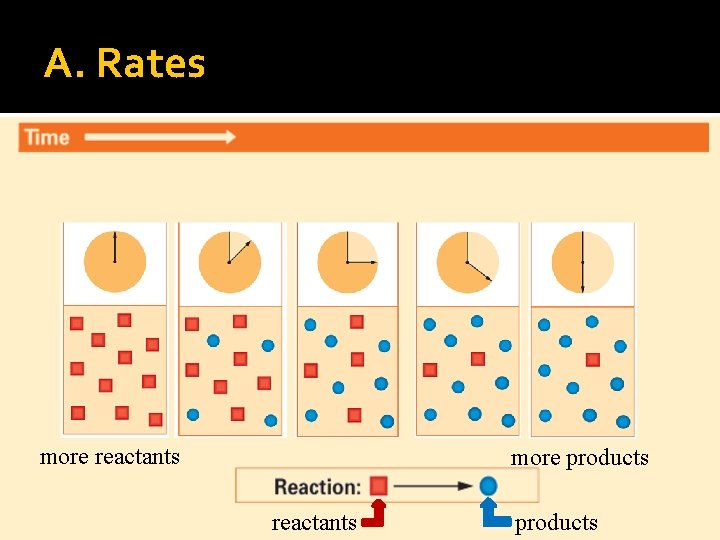
A. Rates more reactants more products reactants products
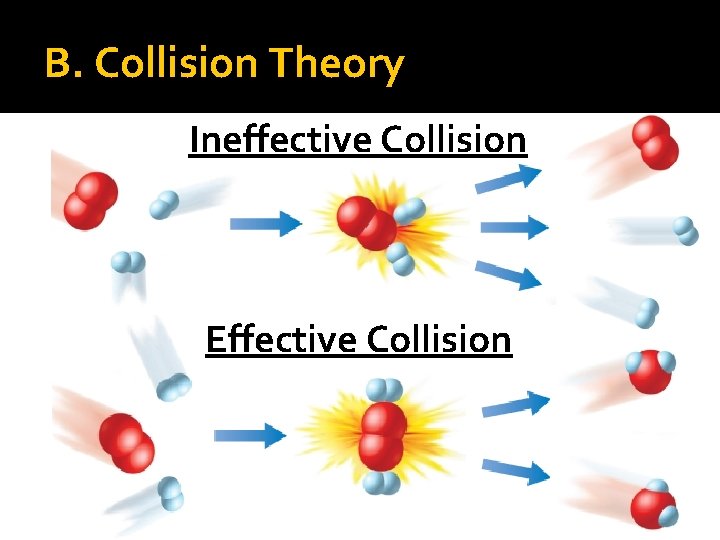
B. Collision Theory Ineffective Collision Effective Collision

B. Collision Theory For reaction to occur: reactants must collide with proper orientation with enough energy needed to react called Activation Energy (Eact)
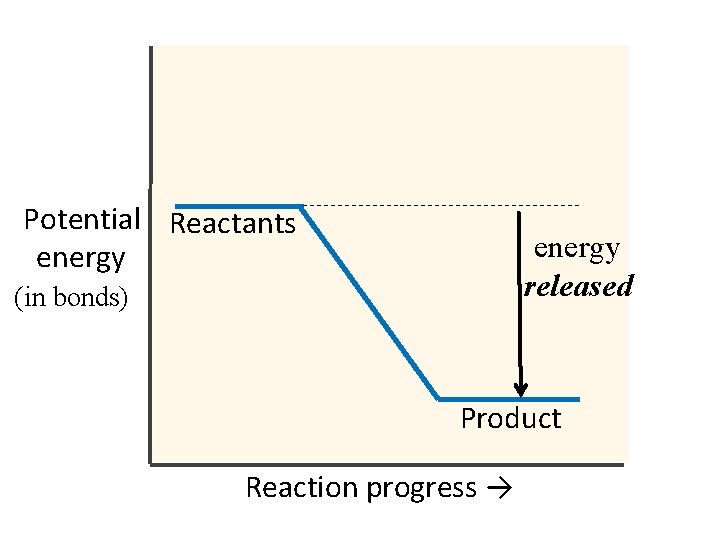
Potential Reactants energy released (in bonds) Product Reaction progress →
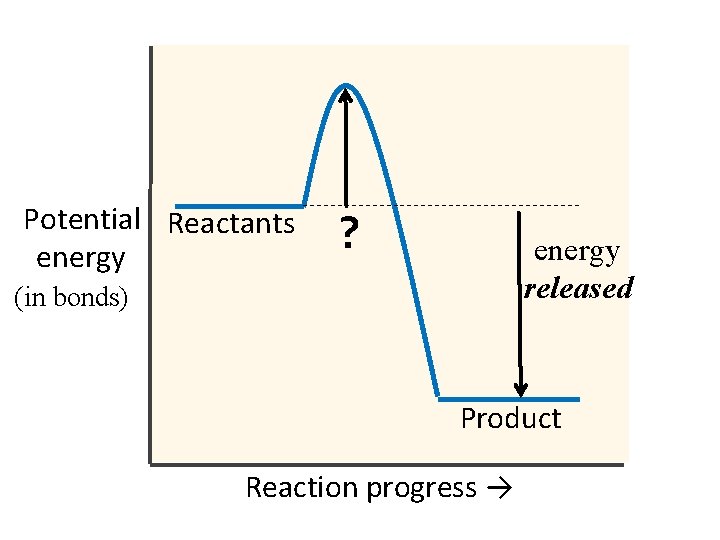
Potential Reactants energy ? energy released (in bonds) Product Reaction progress →
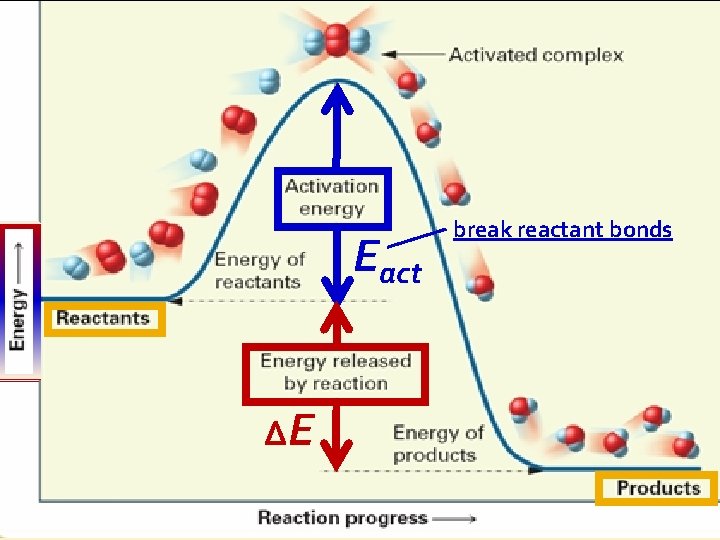
B. Collision Theory Eact ΔE break reactant bonds
C. 5 Factors Affecting Rates The speed that a reaction takes place can be affected by: Temperature Concentration Surface Area Catalysts Stirring
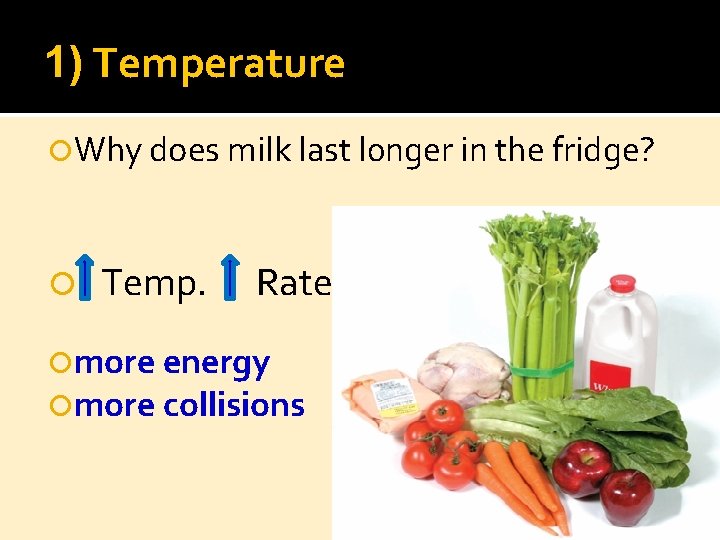
1) Temperature Why does milk last l 0 nger in the fridge? Temp. Rate more energy more collisions
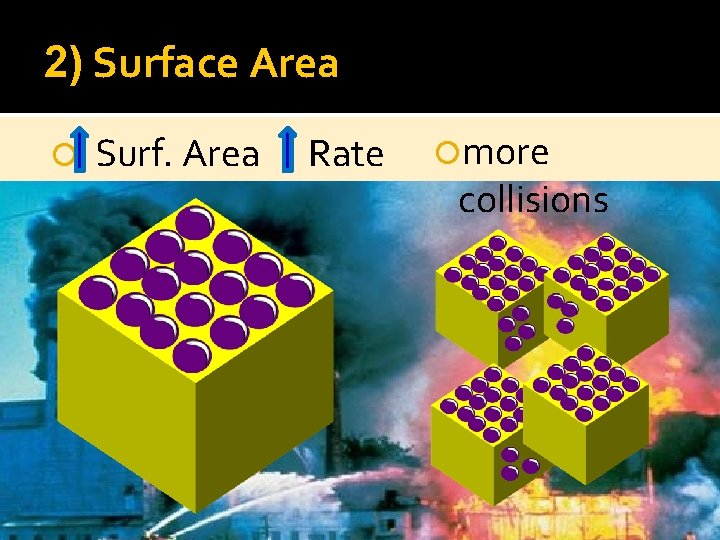
2) Surface Area Surf. Area Rate more collisions

3) Stirring Rate more collisions
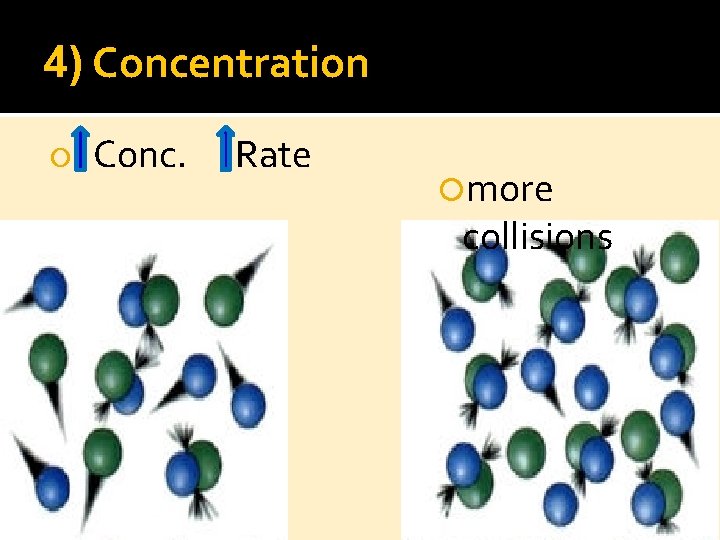
4) Concentration Conc. Rate more collisions
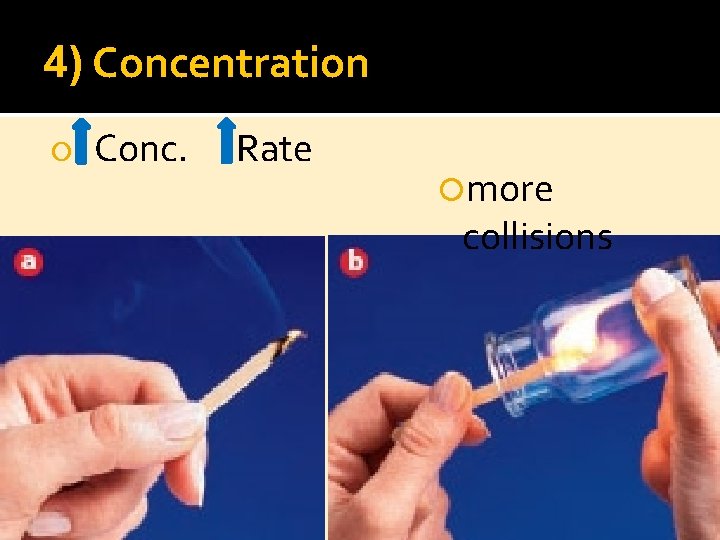
4) Concentration Conc. Rate more collisions

5) Catalysts speed up a reaction without being consumed. lower the activation energy (Ea).
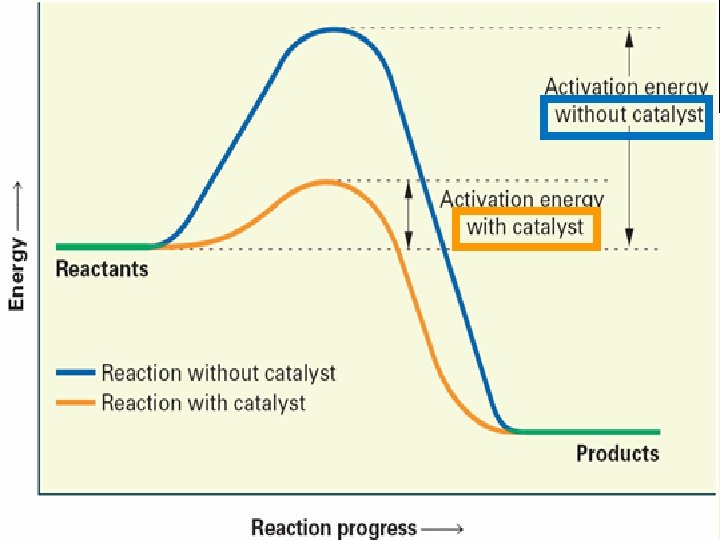
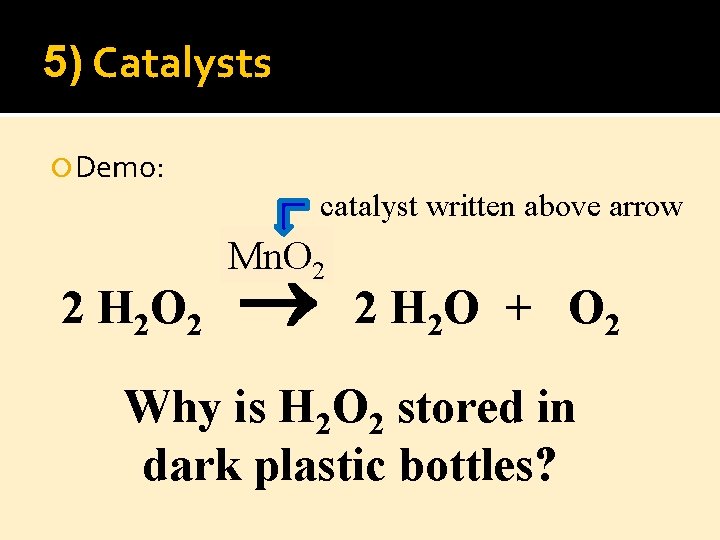
5) Catalysts Demo: catalyst written above arrow 2 H 2 O 2 Mn. O 2 2 H 2 O + O 2 Why is H 2 O 2 stored in dark plastic bottles?

Quick Quiz! 1) An increase in which one of the following will NOT increase the reaction rate? A) temperature B) concentration of reactants C) total mass of reactants D) surface area of reactants

Quick Quiz. 2) A catalyst speeds up a reaction by A) lowering the activation energy. B) increasing the temperature C) permanently changing in a reaction D) supplying energy to a reaction
- Slides: 20