Reaction Mechanisms Free Radical Substitution Monochlorination of methane

Reaction Mechanisms: Free Radical Substitution
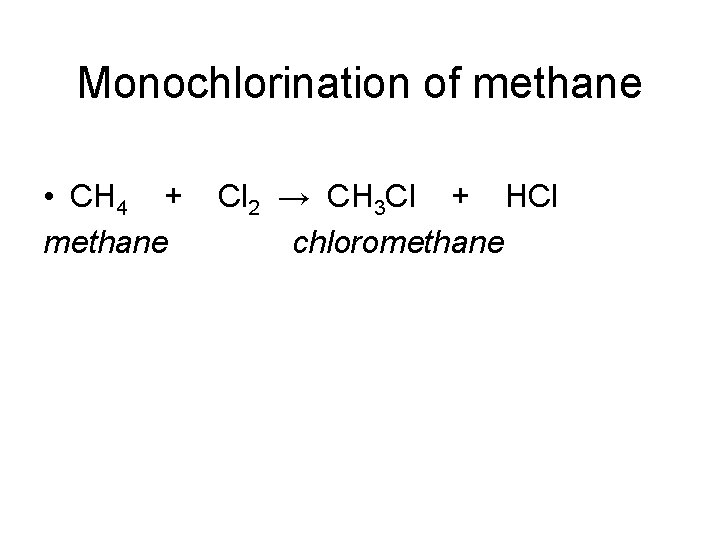
Monochlorination of methane • CH 4 + methane Cl 2 → CH 3 Cl + HCl chloromethane
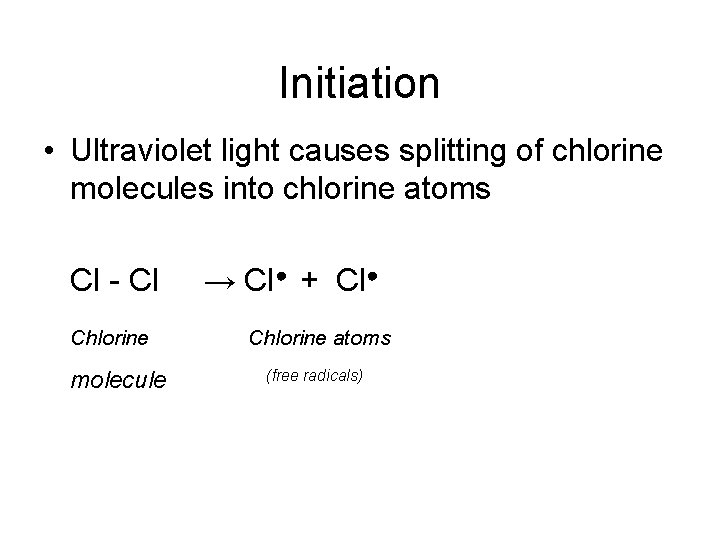
Initiation • Ultraviolet light causes splitting of chlorine molecules into chlorine atoms Cl - Cl Chlorine molecule → Cl● + Cl● Chlorine atoms (free radicals)

Propagation CH 4 methane + CH 3● + Cl● = CH 3● Methyl radical Cl 2 = CH 3 Cl Chloromethane + HCl Hydrogen chloride + Cl ● Chlorine atom free to react with another methane molecule
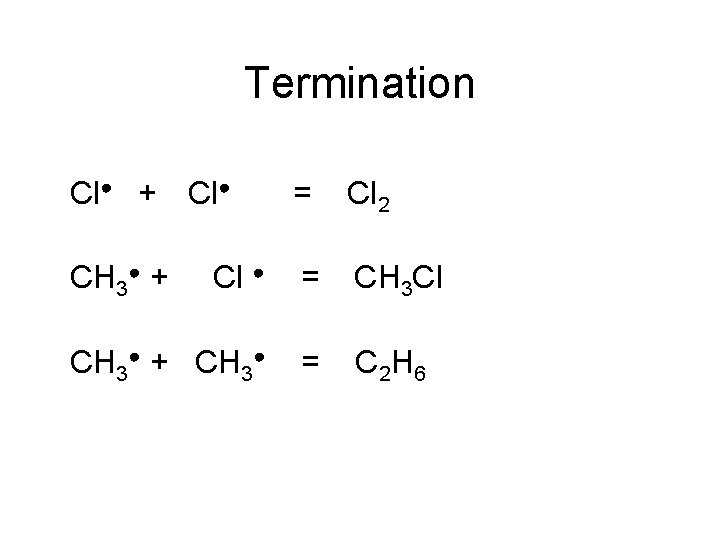
Termination Cl● + = Cl 2 Cl ● = CH 3 Cl CH 3● + CH 3● = C 2 H 6 CH 3● + Cl●

Evidence for this mechanism • Use of ultraviolet light even for a very short period causes a chain reaction • Formation of trace quantities of ethane • Reaction speeded up by sources of free radicals such as tetraethyl lead

Monochlorination of ethane • C 2 H 6 + ethane Cl 2 → C 2 H 5 Cl + chloroethane HCl
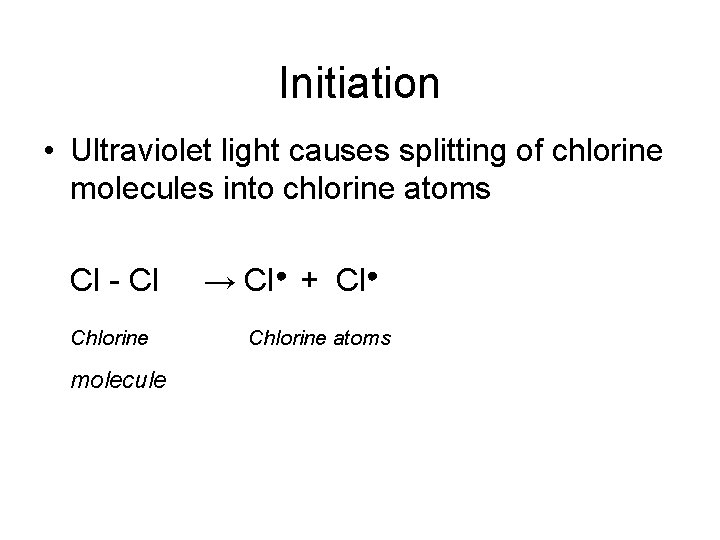
Initiation • Ultraviolet light causes splitting of chlorine molecules into chlorine atoms Cl - Cl Chlorine molecule → Cl● + Cl● Chlorine atoms
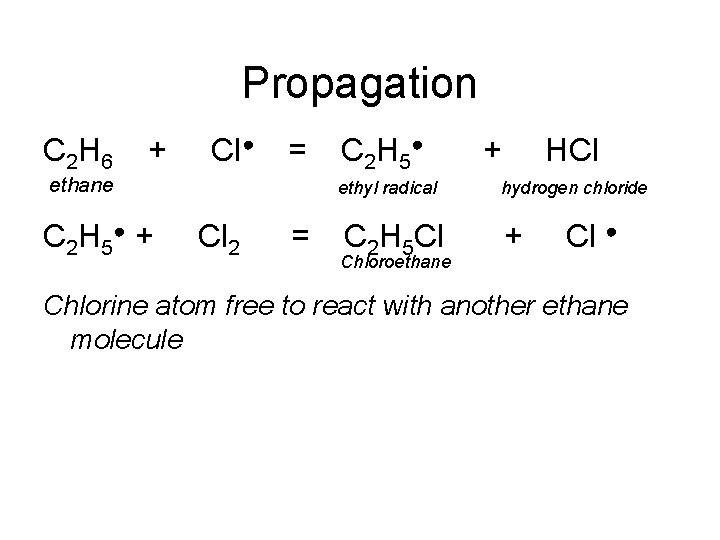
Propagation C 2 H 6 + Cl● = ethane C 2 H 5● + C 2 H 5● ethyl radical Cl 2 = C 2 H 5 Cl Chloroethane + HCl hydrogen chloride + Cl ● Chlorine atom free to react with another ethane molecule
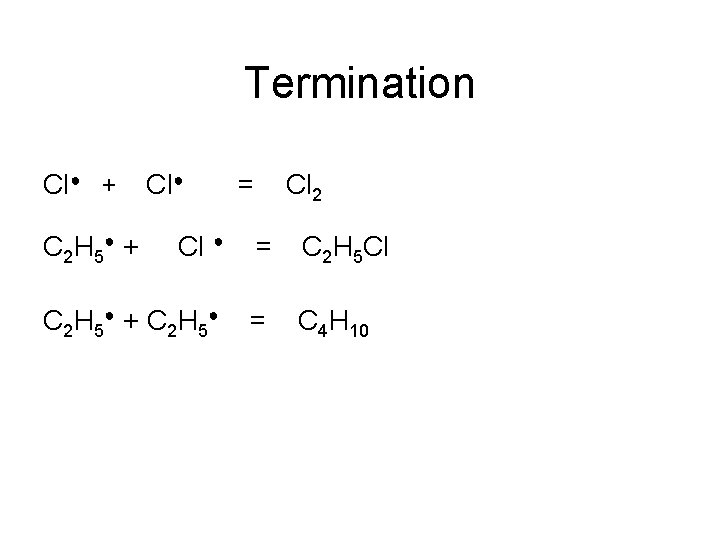
Termination Cl● + C 2 H 5● + Cl● = Cl 2 Cl ● = C 2 H 5 Cl C 2 H 5● + C 2 H 5● = C 4 H 10

Evidence for this mechanism • Use of ultraviolet light even for a very short period causes a chain reaction • Formation of trace quantities of butane • Reaction speeded up by sources of free radicals such as tetraethyl lead
- Slides: 11