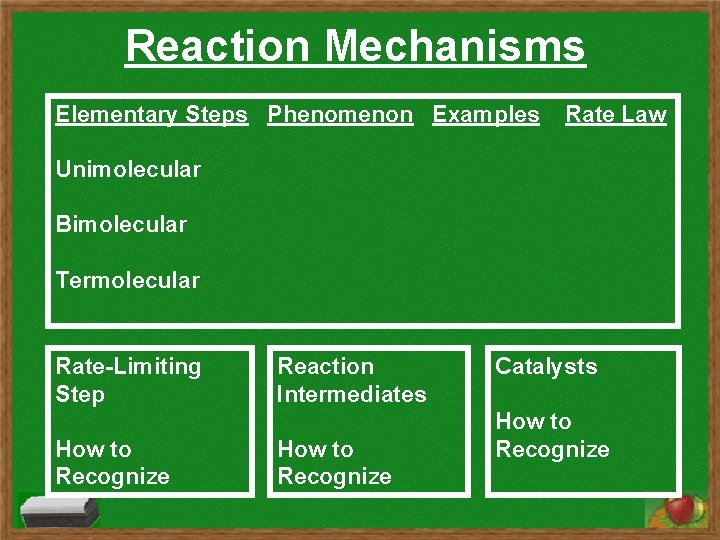
Reaction Mechanisms Elementary Steps Phenomenon Examples Rate Law






- Slides: 19

Reaction Mechanisms Elementary Steps Phenomenon Examples Rate Law Unimolecular Bimolecular Termolecular Rate-Limiting Step How to Recognize Reaction Intermediates How to Recognize Catalysts How to Recognize

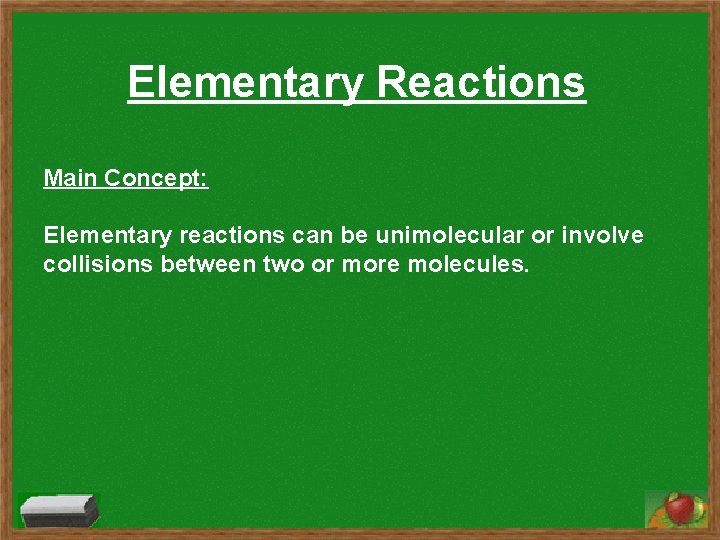
Elementary Reactions Main Concept: Elementary reactions can be unimolecular or involve collisions between two or more molecules.

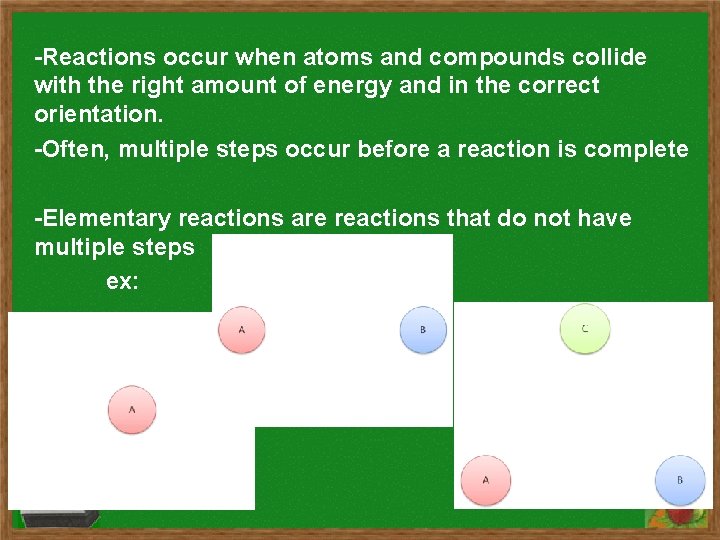
-Reactions occur when atoms and compounds collide with the right amount of energy and in the correct orientation. -Often, multiple steps occur before a reaction is complete -Elementary reactions are reactions that do not have multiple steps ex:

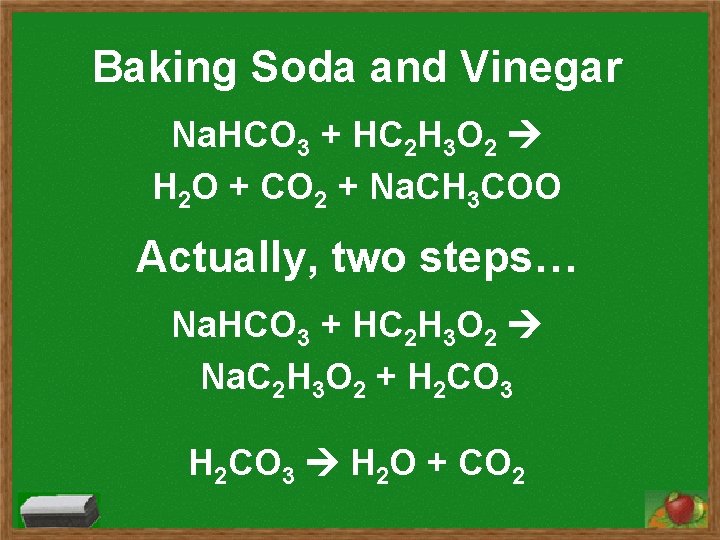
Baking Soda and Vinegar Na. HCO 3 + HC 2 H 3 O 2 H 2 O + CO 2 + Na. CH 3 COO Actually, two steps… Na. HCO 3 + HC 2 H 3 O 2 Na. C 2 H 3 O 2 + H 2 CO 3 H 2 O + CO 2

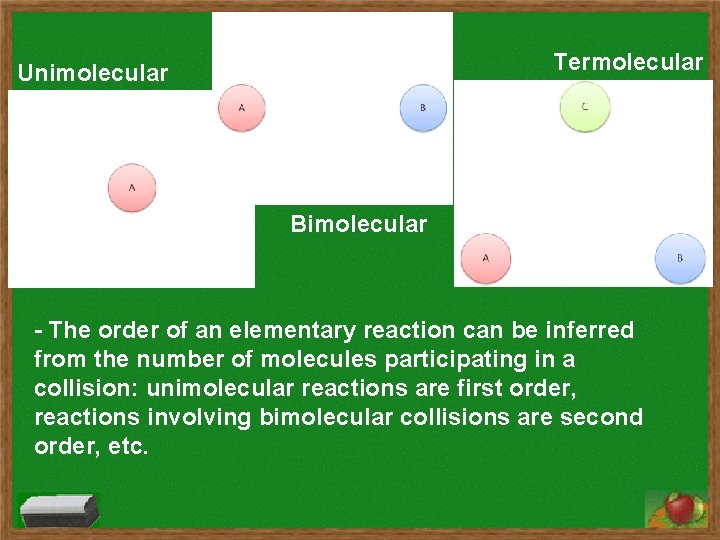
Termolecular Unimolecular Bimolecular - The order of an elementary reaction can be inferred from the number of molecules participating in a collision: unimolecular reactions are first order, reactions involving bimolecular collisions are second order, etc.

- Elementary reactions involving the simultaneous collision of three particles are rare Termolecular

Multistep Reactions Main Concept: The mechanism of a multistep reaction consists of a series of elementary reactions that add up to the overall reaction.

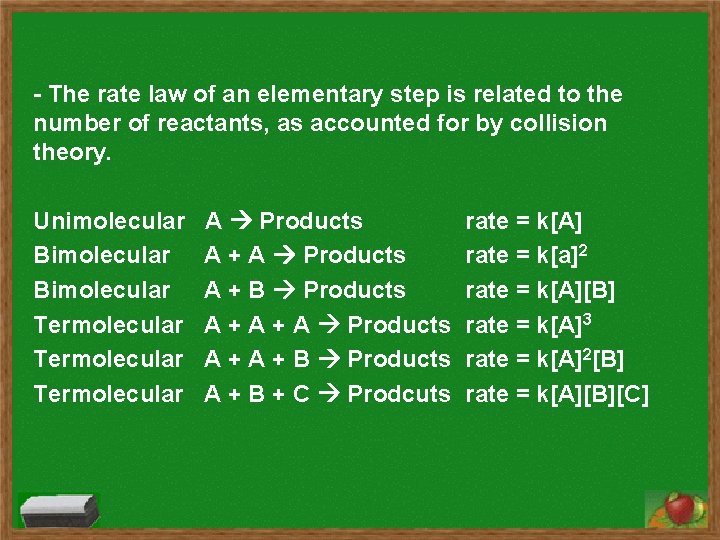
- The rate law of an elementary step is related to the number of reactants, as accounted for by collision theory. Unimolecular Bimolecular Termolecular A Products A + B Products A + B + C Prodcuts rate = k[A] rate = k[a]2 rate = k[A][B] rate = k[A]3 rate = k[A]2[B] rate = k[A][B][C]

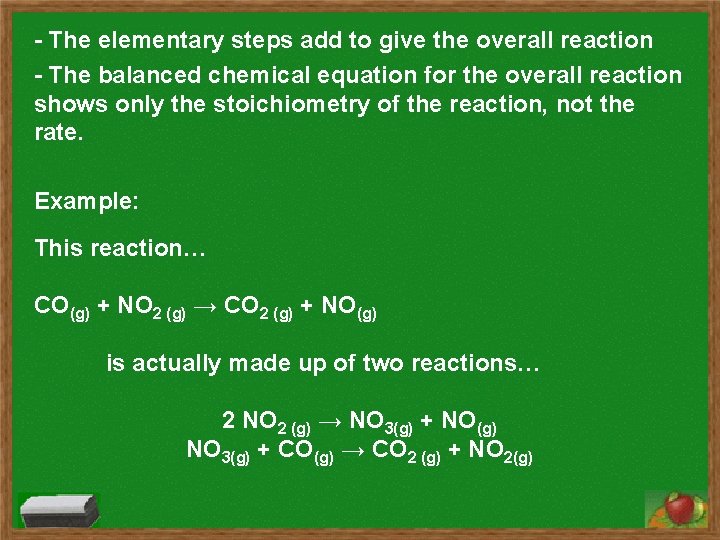
- The elementary steps add to give the overall reaction - The balanced chemical equation for the overall reaction shows only the stoichiometry of the reaction, not the rate. Example: This reaction… CO(g) + NO 2 (g) → CO 2 (g) + NO(g) is actually made up of two reactions… 2 NO 2 (g) → NO 3(g) + NO(g) NO 3(g) + CO(g) → CO 2 (g) + NO 2(g)

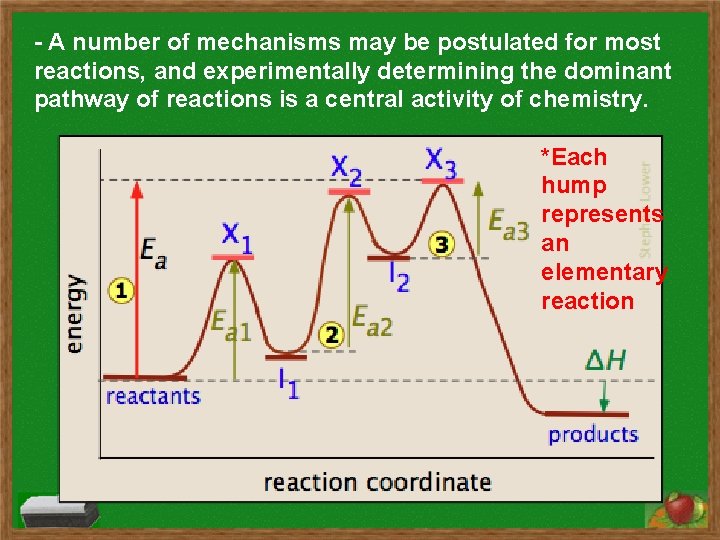
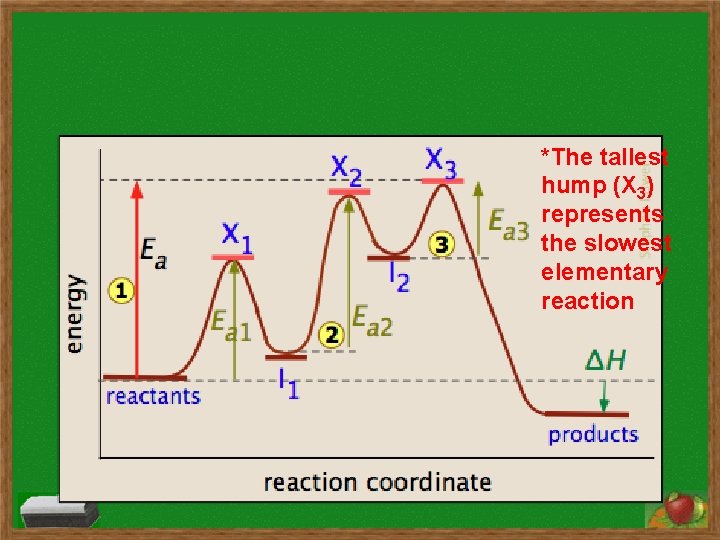
- A number of mechanisms may be postulated for most reactions, and experimentally determining the dominant pathway of reactions is a central activity of chemistry. *Each hump represents an elementary reaction

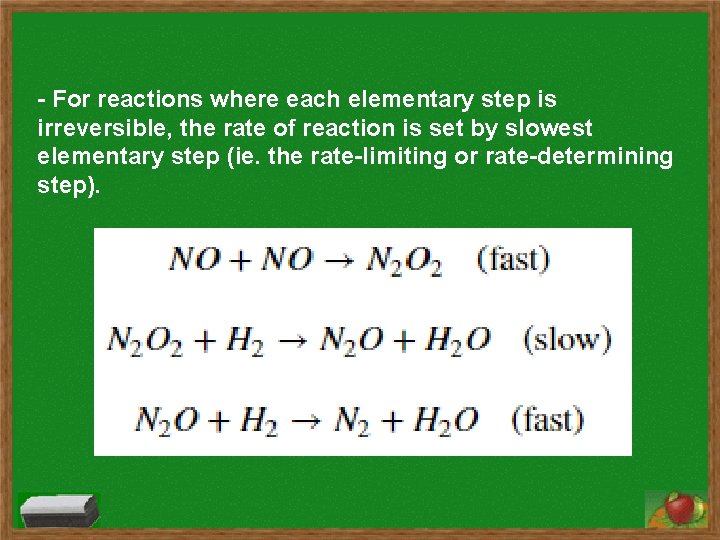
Rate Limiting Step Main Concept: In many reactions, the rate is set up by the slowest elementary reaction, or rate-limiting step.

- For reactions where each elementary step is irreversible, the rate of reaction is set by slowest elementary step (ie. the rate-limiting or rate-determining step).

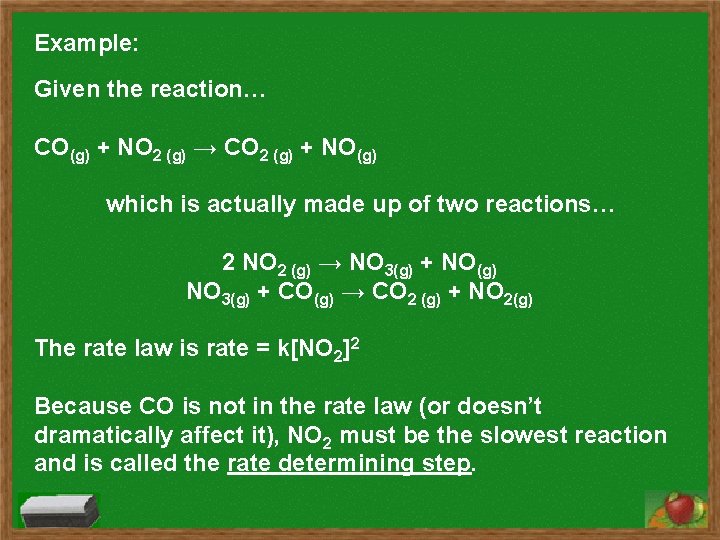
Example: Given the reaction… CO(g) + NO 2 (g) → CO 2 (g) + NO(g) which is actually made up of two reactions… 2 NO 2 (g) → NO 3(g) + NO(g) NO 3(g) + CO(g) → CO 2 (g) + NO 2(g) The rate law is rate = k[NO 2]2 Because CO is not in the rate law (or doesn’t dramatically affect it), NO 2 must be the slowest reaction and is called the rate determining step.

*The tallest hump (X 3) represents the slowest elementary reaction


Reaction Intermediates Main Concept: Reaction intermediates, which are formed during the reaction but not present in the overall reaction, play an important role in multistep reactions.

- A reaction intermediate is produced by some elementary steps and consumed by others; it is present only while a reaction is occurring - Experimental detection of a reaction intermediate is a way to build evidence in support of one reaction mechanism over an alternative mechanism - Catalysts appear at the beginning of reaction steps and again at the end of the reaction steps, but not in the overall reaction

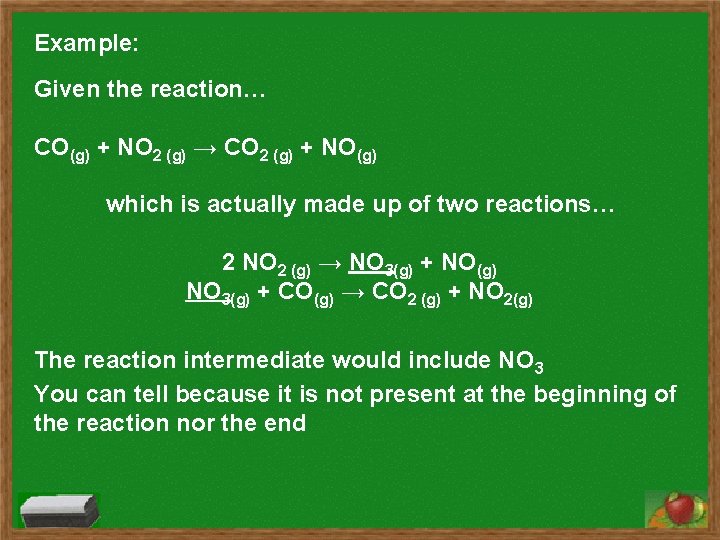
Example: Given the reaction… CO(g) + NO 2 (g) → CO 2 (g) + NO(g) which is actually made up of two reactions… 2 NO 2 (g) → NO 3(g) + NO(g) NO 3(g) + CO(g) → CO 2 (g) + NO 2(g) The reaction intermediate would include NO 3 You can tell because it is not present at the beginning of the reaction nor the end

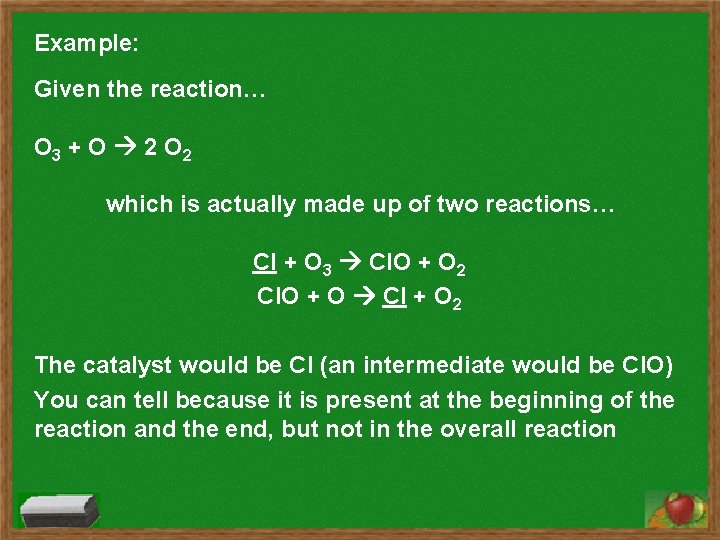
Example: Given the reaction… O 3 + O 2 O 2 which is actually made up of two reactions… Cl + O 3 Cl. O + O 2 Cl. O + O Cl + O 2 The catalyst would be Cl (an intermediate would be Cl. O) You can tell because it is present at the beginning of the reaction and the end, but not in the overall reaction