Reaction Mechanisms and evidence for Heterolytic Fission Step







- Slides: 12

Reaction Mechanisms and evidence for Heterolytic Fission

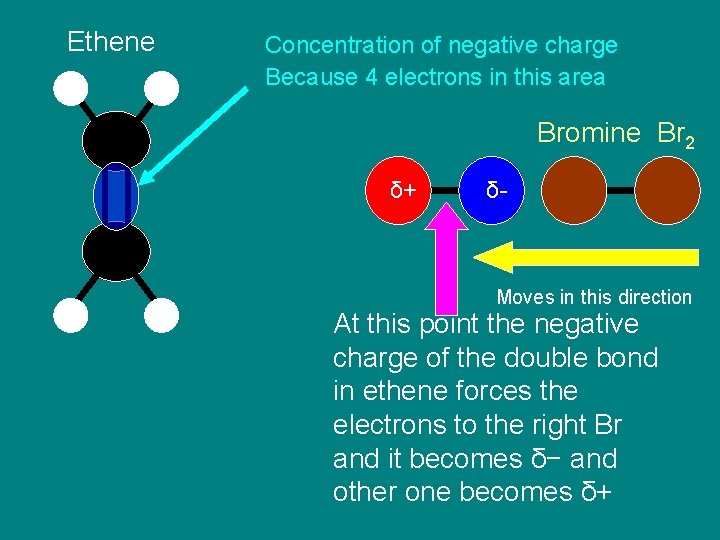
Step 1 Polarising of the bond in bromine

Ethene Concentration of negative charge Because 4 electrons in this area Bromine Br 2 δ+ δ- Moves in this direction At this point the negative charge of the double bond in ethene forces the electrons to the right Br and it becomes δ− and other one becomes δ+

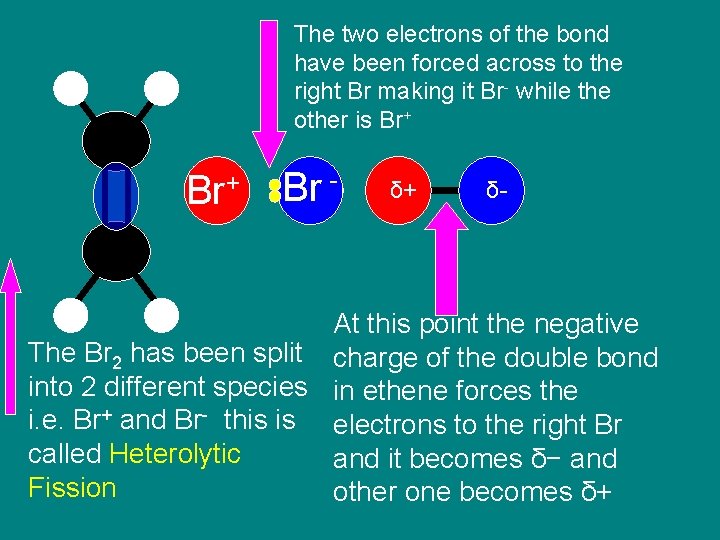
Step 2 Heterolytic Fission Occurs

The two electrons of the bond have been forced across to the right Br making it Br- while the other is Br+ Br - δ+ δ- At this point the negative The Br 2 has been split charge of the double bond into 2 different species in ethene forces the i. e. Br+ and Br- this is electrons to the right Br called Heterolytic and it becomes δ− and Fission other one becomes δ+

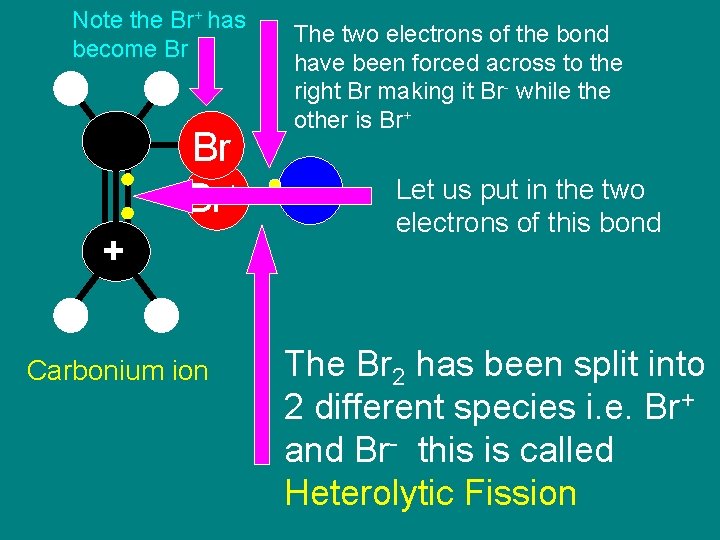
Step 3 Formation of the Carbonium Ion

Note the Br+ has become Br Br Br+ + Carbonium ion The two electrons of the bond have been forced across to the right Br making it Br- while the other is Br+ Br - Let us put in the two electrons of this bond The Br 2 has been split into 2 different species i. e. Br+ and Br- this is called Heterolytic Fission

• At this point a lot of things happen at the same time • The two electrons are pulled to the Br+ • A bond is formed • one of the bonds between the two carbons disappears • The lower carbon becomes +ve because it has lost an electron • The two hydrogens on the upper carbon move to make way for the Br

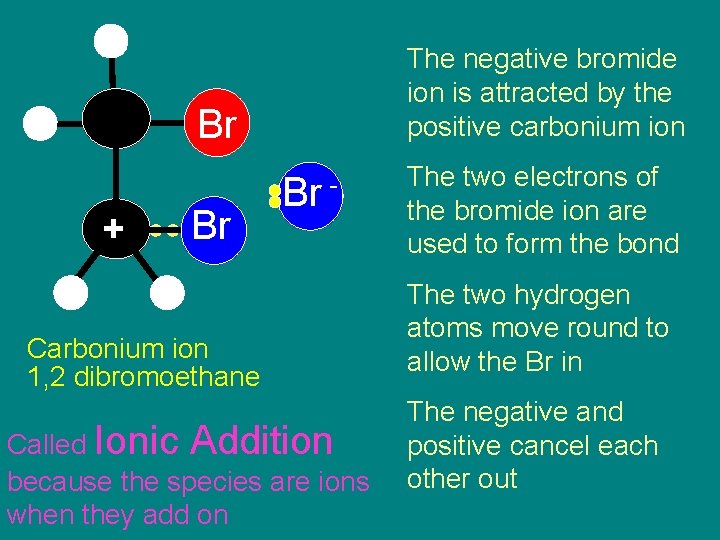
Step 4 Attack on carbonium ion by Br

The negative bromide ion is attracted by the positive carbonium ion Br + Br Br - Carbonium ion 1, 2 dibromoethane Called Ionic Addition because the species are ions when they add on The two electrons of the bromide ion are used to form the bond The two hydrogen atoms move round to allow the Br in The negative and positive cancel each other out

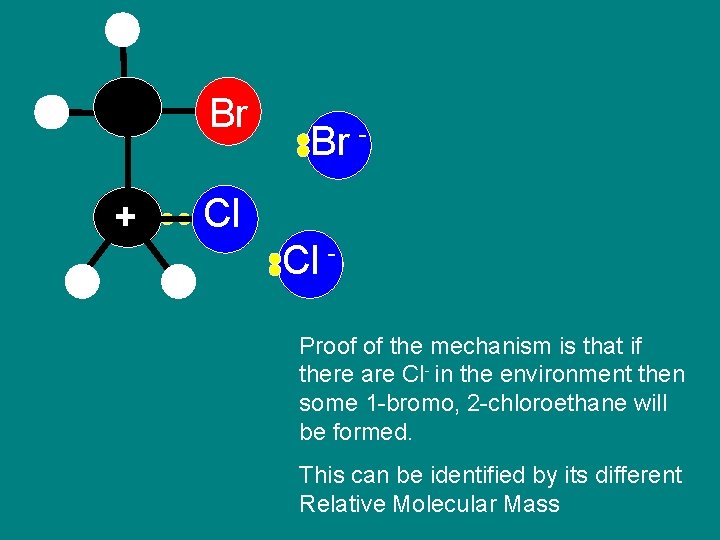
Step 5 Proof of mechanism

Br + Br - Cl Cl Br Proof of the mechanism is that if there are Cl- in the environment then some 1 -bromo, 2 -chloroethane will be formed. This can be identified by its different Relative Molecular Mass