Reaction Mechanisms and Equilibrium The rates of the
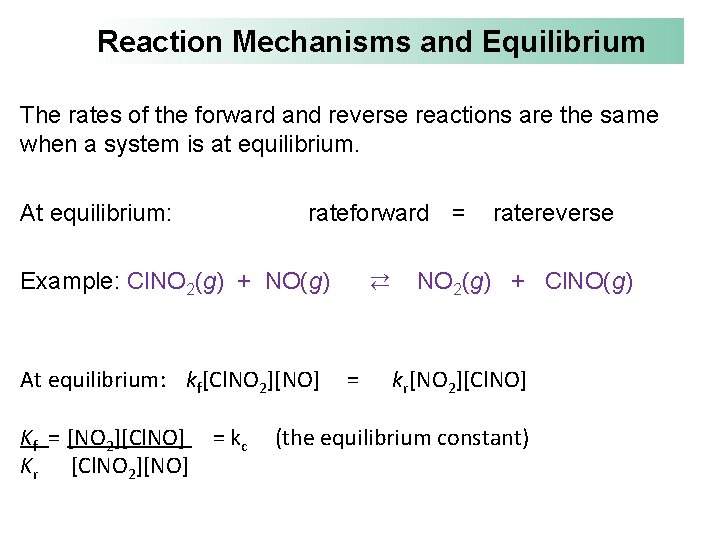
Reaction Mechanisms and Equilibrium The rates of the forward and reverse reactions are the same when a system is at equilibrium. At equilibrium: rateforward = Example: Cl. NO 2(g) + NO(g) At equilibrium: kf[Cl. NO 2][NO] Kf = [NO 2][Cl. NO] = kc Kr [Cl. NO 2][NO] ⇄ = ratereverse NO 2(g) + Cl. NO(g) kr[NO 2][Cl. NO] (the equilibrium constant)

Solving a rate law involving equilibrium 1. Find the slowest step (the rate-determining step) 2. Write a temporary rate law using the slowest step. 3. Write the equilibium expression for the fastforward reversible elementary step. (the one at equilibrium with the double arrow) 4. Substitute the equation from step 3 into the temporary rate law from step 2 so that only reactant are present (no intermediates or products!) and solve.
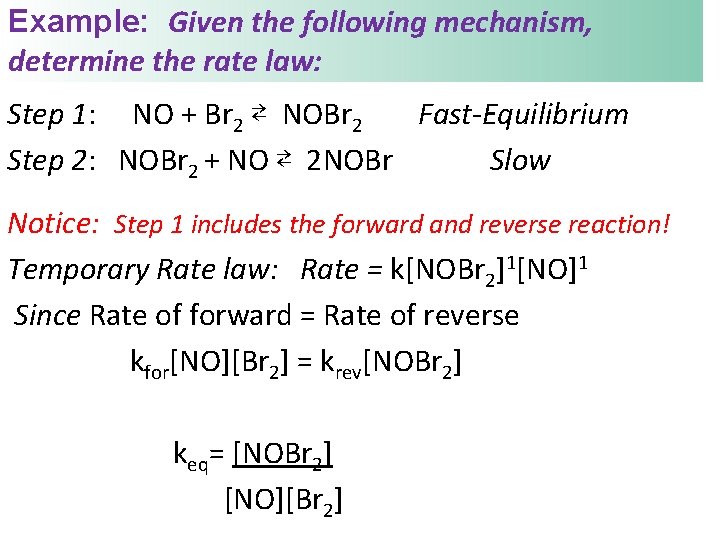
Example: Given the following mechanism, determine the rate law: Step 1: NO + Br 2 ⇄ NOBr 2 Fast-Equilibrium Step 2: NOBr 2 + NO ⇄ 2 NOBr Slow Notice: Step 1 includes the forward and reverse reaction! Temporary Rate law: Rate = k[NOBr 2]1[NO]1 Since Rate of forward = Rate of reverse kfor[NO][Br 2] = krev[NOBr 2] keq= [NOBr 2] [NO][Br 2]

Use the equilibrium expression to solve for the product on the equilibrium step: keq = [NOBr 2] [NO][Br 2] NOBr 2 is an intermediate, solve for it [NOBr 2] = k[NO][Br 2] Substitute into temporary law: Rate = k[NOBr 2]1[NO]1 Rate = k[NO]1[Br 2]1[NO]1 Is this a valid mechanism? Rate = k[Br 2]1[NO]2 Yes
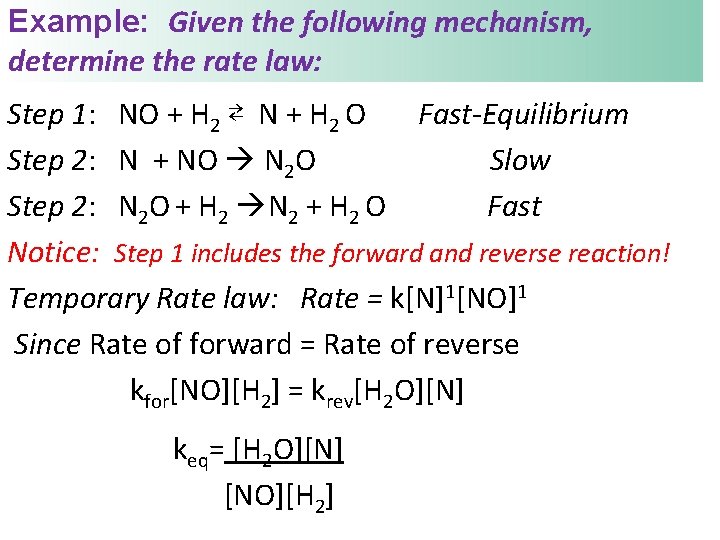
Example: Given the following mechanism, determine the rate law: Step 1: NO + H 2 ⇄ N + H 2 O Fast-Equilibrium Step 2: N + NO N 2 O Slow Step 2: N 2 O + H 2 N 2 + H 2 O Fast Notice: Step 1 includes the forward and reverse reaction! Temporary Rate law: Rate = k[N]1[NO]1 Since Rate of forward = Rate of reverse kfor[NO][H 2] = krev[H 2 O][N] keq= [H 2 O][N] [NO][H 2]

Use the equilibrium expression to solve for the product on the equilibrium step: keq = [H 2 O][N] [NO][H 2] • N is an intermediate , solve for it & substitute [N] = k[NO][H 2] [H 2 O] Substitute into temporary law: Rate = k[N]1[NO]1 Rate = k[NO]1[H 2]1[NO]1 [H 2 O] Is this a valid mechanism? No Rate = k[H 2]1[NO]2 [H 2 O]-1

The end.
- Slides: 7