Reaction Mechanisms and Catalysts Mr Krstovic Grade 12


- Slides: 13

Reaction Mechanisms and Catalysts Mr. Krstovic Grade 12 Chemistry

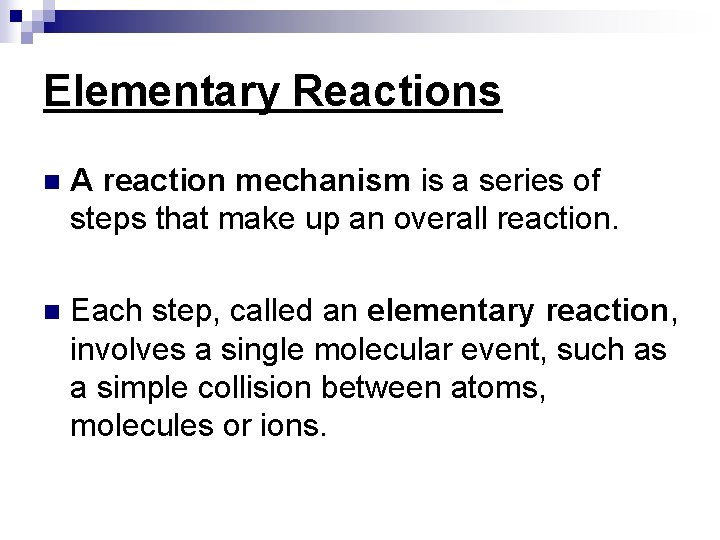
Elementary Reactions n A reaction mechanism is a series of steps that make up an overall reaction. n Each step, called an elementary reaction, involves a single molecular event, such as a simple collision between atoms, molecules or ions.

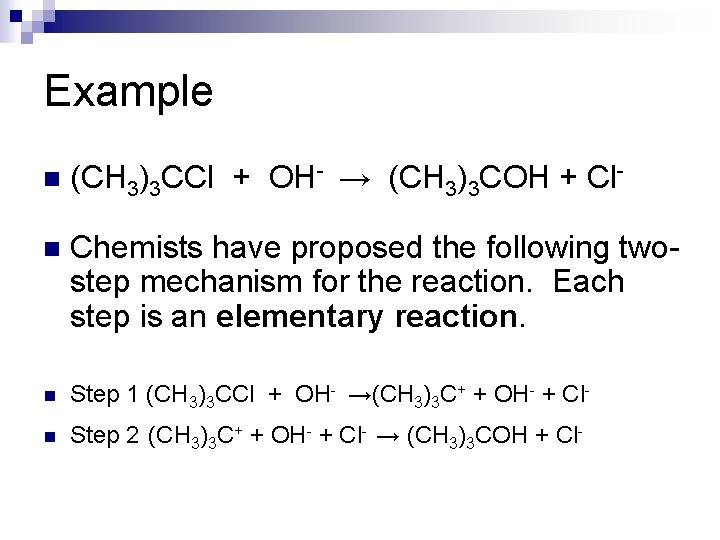
Example n (CH 3)3 CCl + OH- → (CH 3)3 COH + Cl- n Chemists have proposed the following twostep mechanism for the reaction. Each step is an elementary reaction. n Step 1 (CH 3)3 CCl + OH- →(CH 3)3 C+ + OH- + Cl- n Step 2 (CH 3)3 C+ + OH- + Cl- → (CH 3)3 COH + Cl-


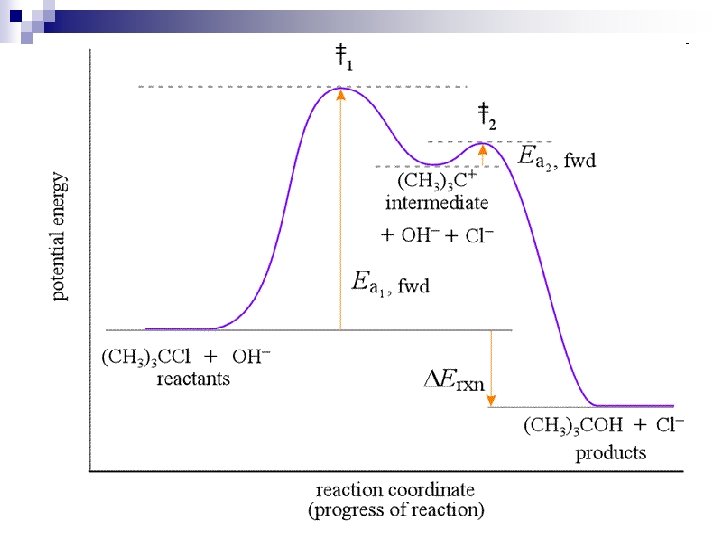
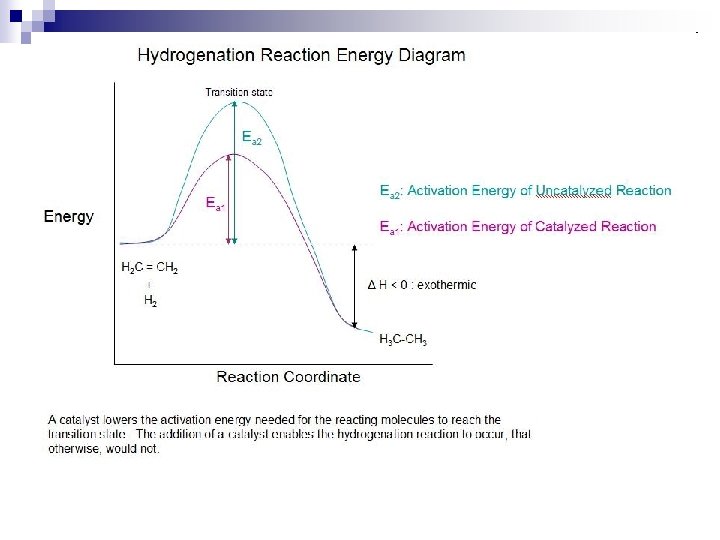
Explaining the Potential Energy Diagram: n n Ea = activation energy: minimum energy required for a reaction to proceed Ea (forward) vs. Ea (reverse) Activated Complex forms at the ‘top’ of each ‘hump’ – these are partially broken and partially formed bonds (not shown in the reaction mechanism) Reaction Intermediates: short-lived, highly reactive molecules/atoms/ions that go on to react further (part of the reaction mechanism)

Proposing and Evaluating Mechanisms n When chemists propose a mechanism, they must satisfy the following criteria: 1. The equation for the elementary steps must combine to give the equation for the overall reaction The proposed elementary steps must be reasonable The mechanism must support the experimentally determined rate law 2. 3.

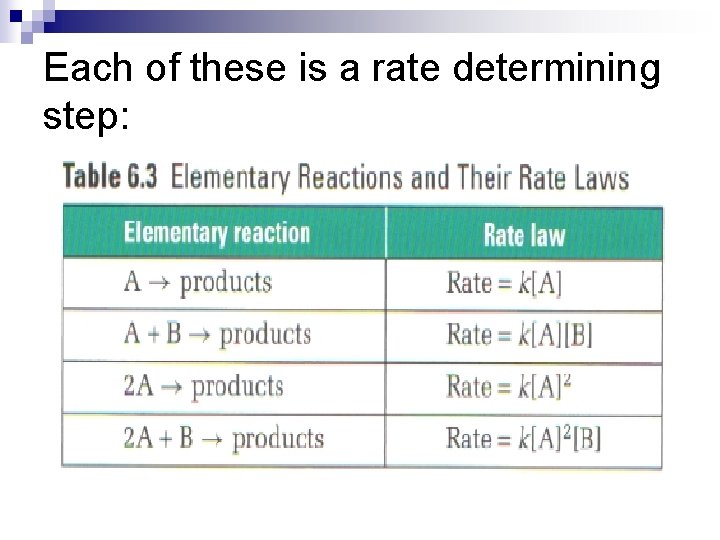
The Rate Determining Step n Elementary reactions in mechanisms all have different rates. n Usually one elementary reaction, called the ratedetermining step (RDS), is much slower. n Hence, it determines the overall rate. The rate law can be written directly using balanced coefficients in the RDS – these from the exponents in the rate law!

Each of these is a rate determining step:

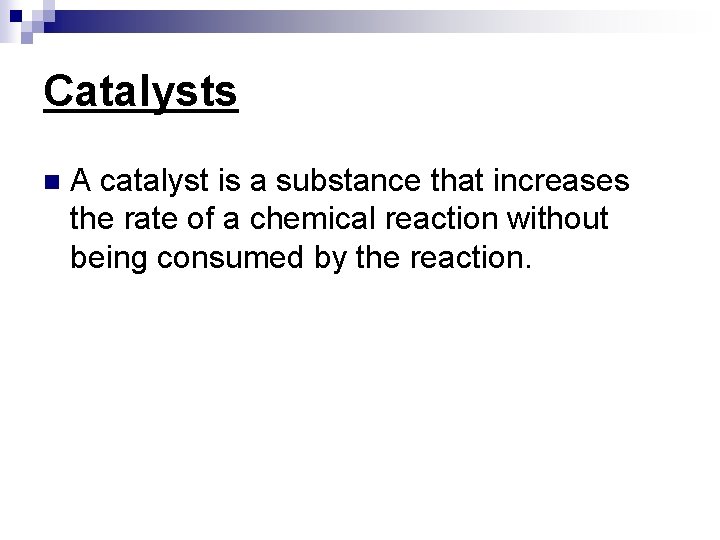
Catalysts n A catalyst is a substance that increases the rate of a chemical reaction without being consumed by the reaction.

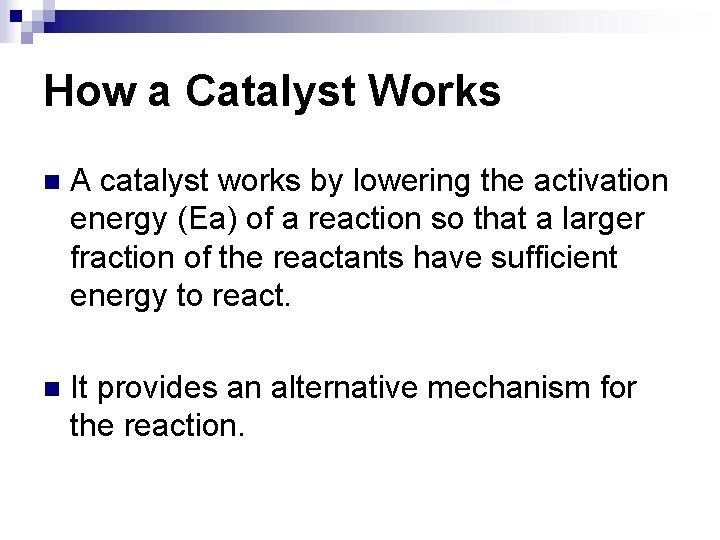
How a Catalyst Works n A catalyst works by lowering the activation energy (Ea) of a reaction so that a larger fraction of the reactants have sufficient energy to react. n It provides an alternative mechanism for the reaction.


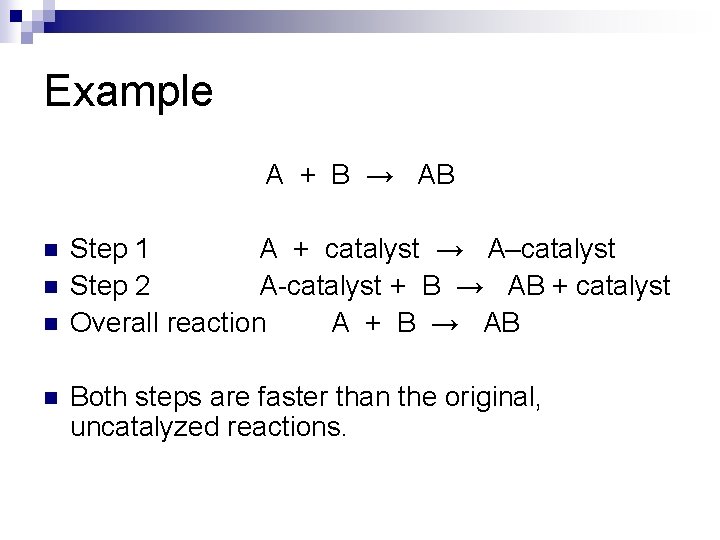
Example A + B → AB n n Step 1 A + catalyst → A–catalyst Step 2 A-catalyst + B → AB + catalyst Overall reaction A + B → AB Both steps are faster than the original, uncatalyzed reactions.

Homework n Please do the following questions from the text: