Reaction mechanism of iterative minimal polyketide synthases PKS

- Slides: 27

Reaction mechanism of iterative minimal polyketide synthases (PKS) Polyketide synthases are multidomain enzymes that catalyze the condensation of ketide units (starter unit and extender units) resulting in the formation of polyketides. The reaction is driven by decarboxylation of the extender unit during condensation, which is also known as a Claisen condensation. The motivation for making this animation was that many of our students struggled with understanding how the different substrates and products were moved around inside the PKS, during biosynthesis. The following slides shows the conceptual reaction mechanism and is not correct in chemical terms with respect to the flow of electrons. Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

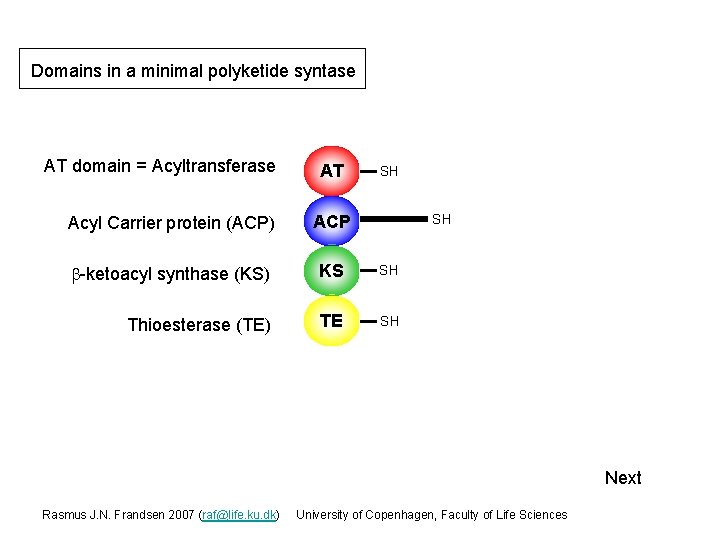
Domains in a minimal polyketide syntase AT domain = Acyltransferase AT SH Acyl Carrier protein (ACP) ACP b-ketoacyl synthase (KS) KS SH Thioesterase (TE) TE SH SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

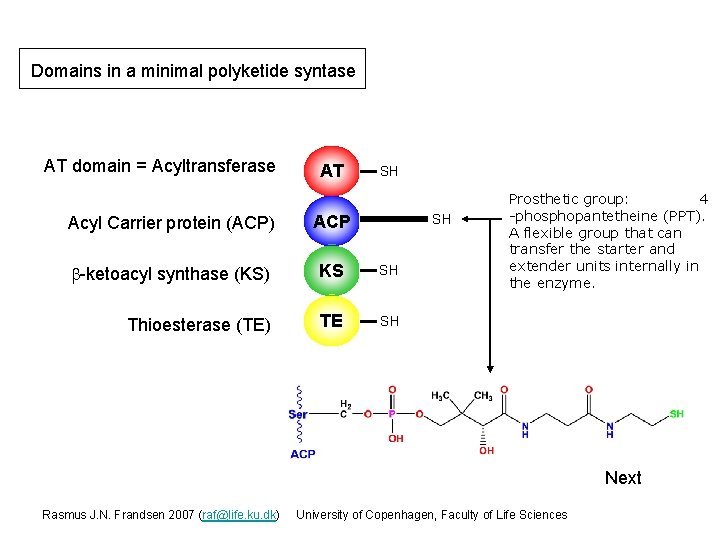
Domains in a minimal polyketide syntase AT domain = Acyltransferase AT SH Acyl Carrier protein (ACP) ACP b-ketoacyl synthase (KS) KS SH Thioesterase (TE) TE SH SH Prosthetic group: 4 -phosphopantetheine (PPT). A flexible group that can transfer the starter and extender units internally in the enzyme. Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

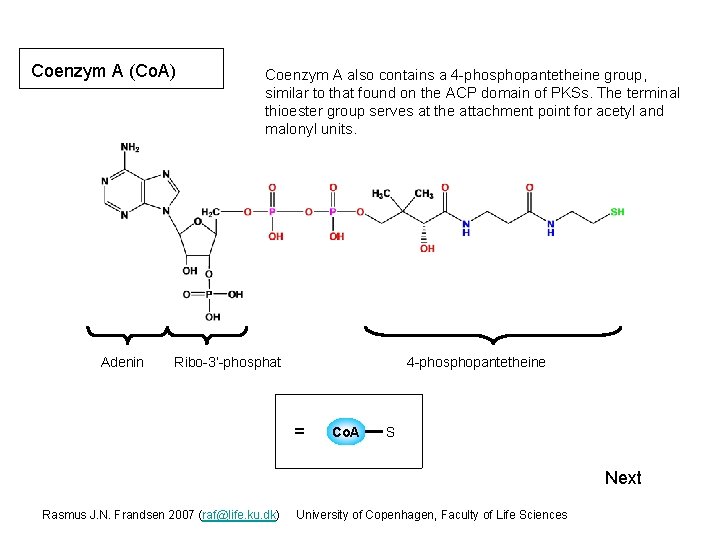
Coenzym A (Co. A) Adenin Coenzym A also contains a 4 -phosphopantetheine group, similar to that found on the ACP domain of PKSs. The terminal thioester group serves at the attachment point for acetyl and malonyl units. Ribo-3’-phosphat 4 -phosphopantetheine = Co. A S Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

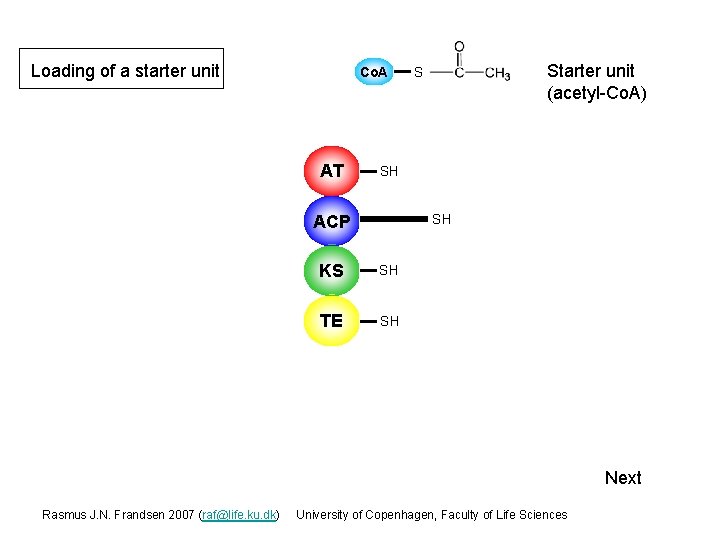
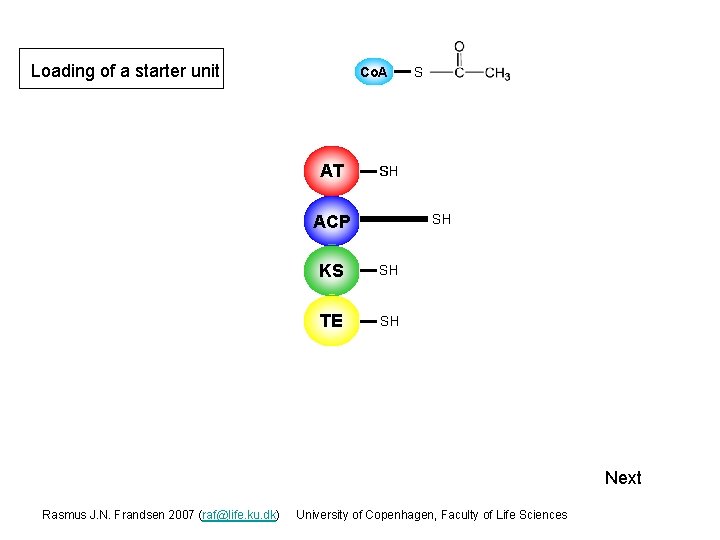
Loading of a starter unit Co. A AT Starter unit (acetyl-Co. A) S SH ACP SH KS SH TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

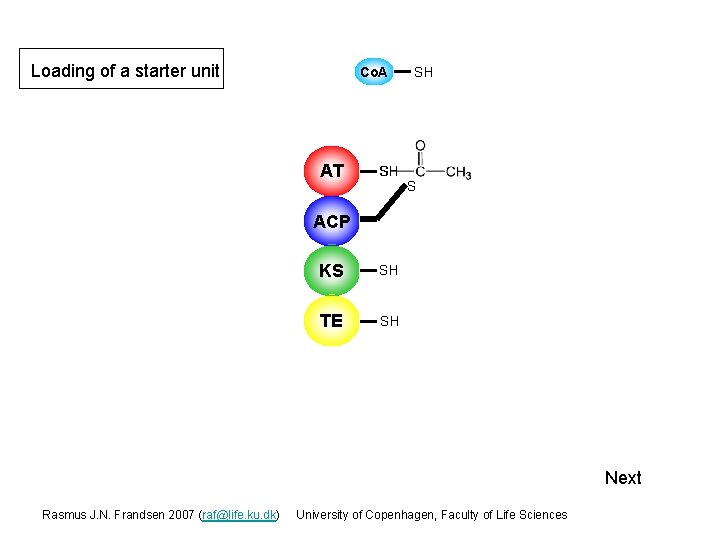
Loading of a starter unit Co. A AT S S SH ACP SH KS SH TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

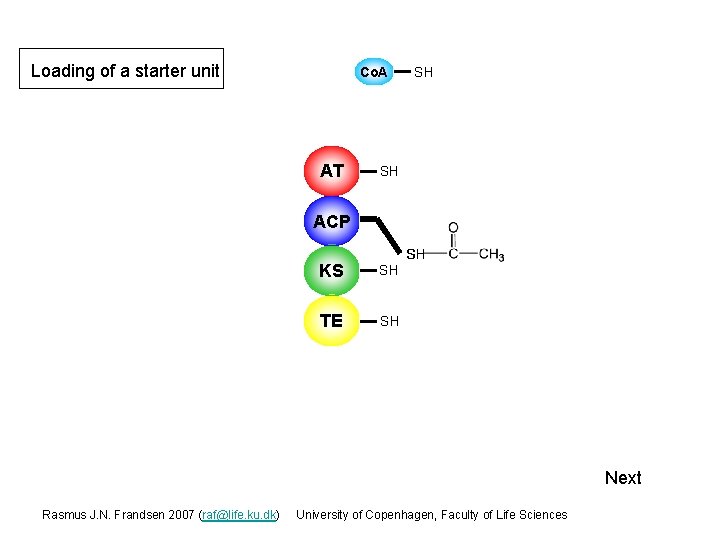
Loading of a starter unit Co. A AT SH S ACP KS SH TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

Loading of a starter unit Co. A AT SH SH ACP SH S KS S SH TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

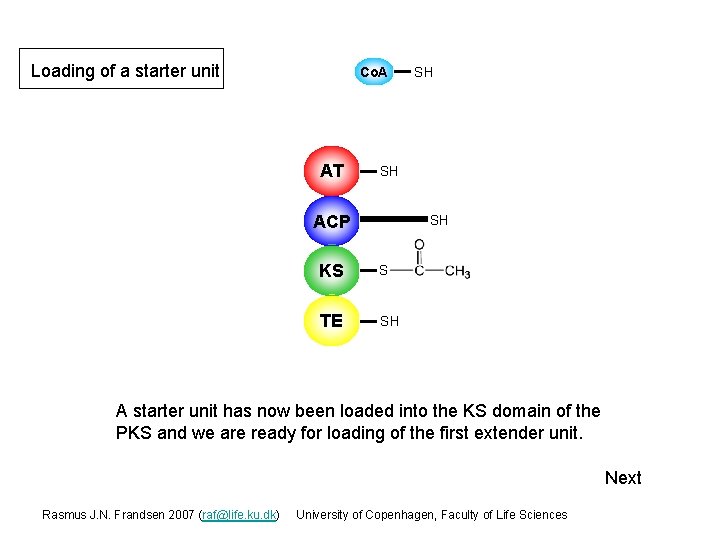
Loading of a starter unit Co. A AT SH SH ACP SH KS S TE SH A starter unit has now been loaded into the KS domain of the PKS and we are ready for loading of the first extender unit. Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

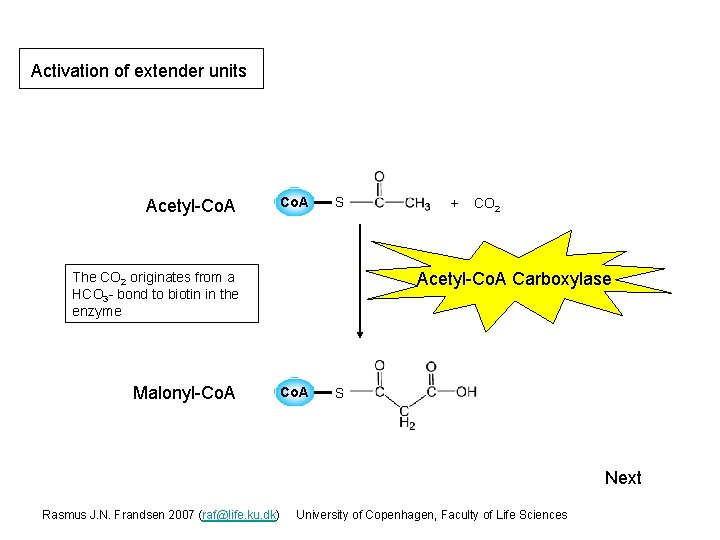
Activation of extender units Acetyl-Co. A S CO 2 Acetyl-Co. A Carboxylase The CO 2 originates from a HCO 3 - bond to biotin in the enzyme Malonyl-Co. A + Co. A S Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

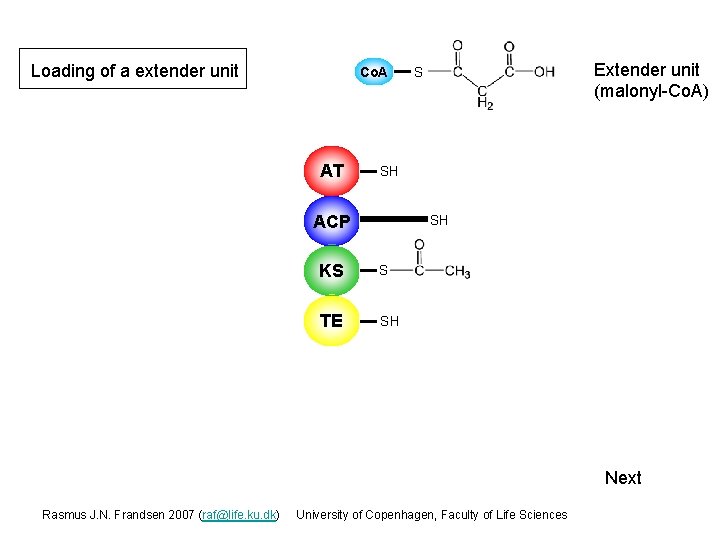
Loading of a extender unit Co. A AT Extender unit (malonyl-Co. A) S SH ACP SH KS S TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

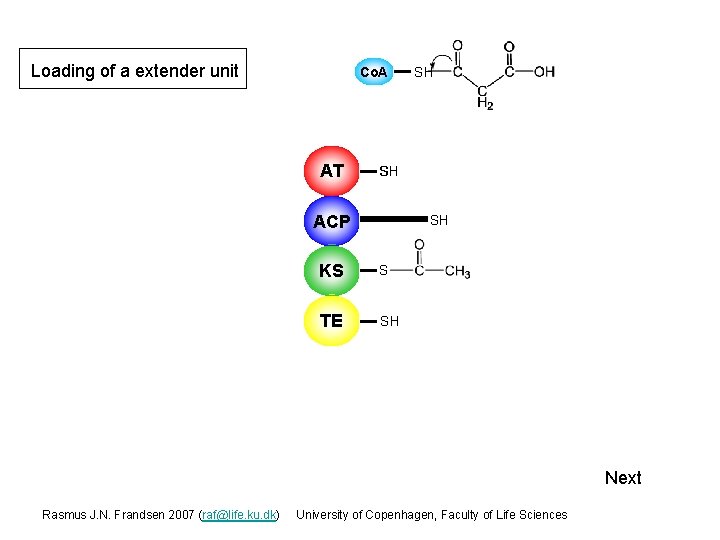
Loading of a extender unit Co. A AT S SH SH ACP SH KS S TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

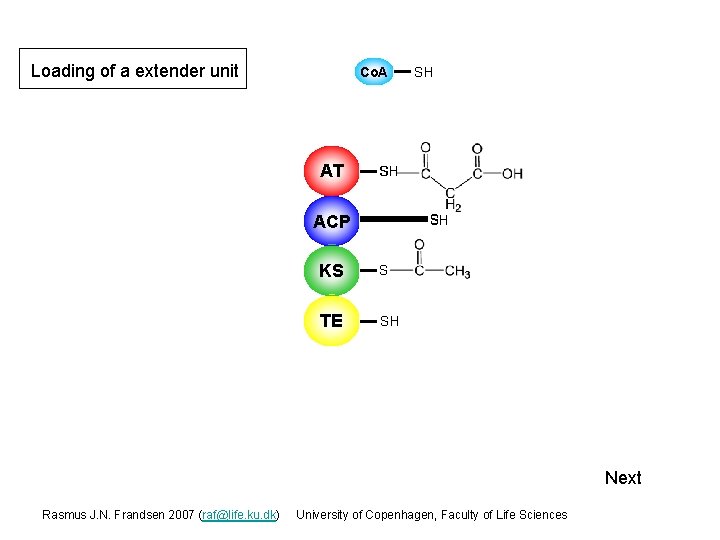
Loading of a extender unit Co. A AT SH SH S ACP S SH KS S TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

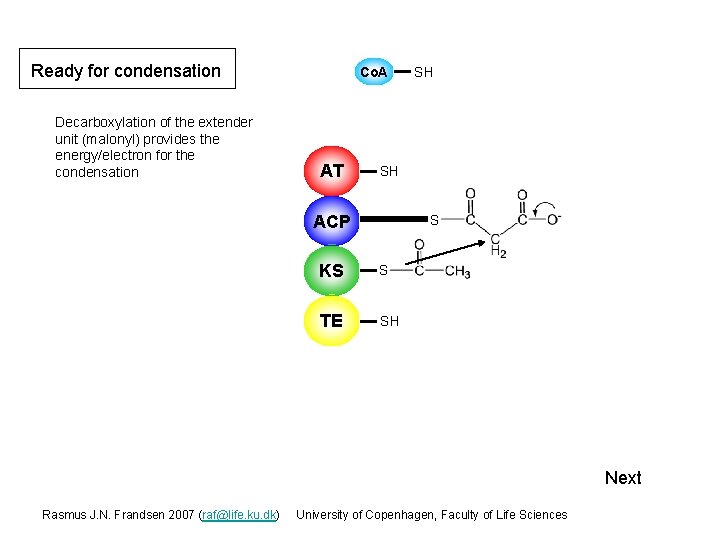
Ready for condensation Decarboxylation of the extender unit (malonyl) provides the energy/electron for the condensation Co. A AT SH SH ACP S KS S TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

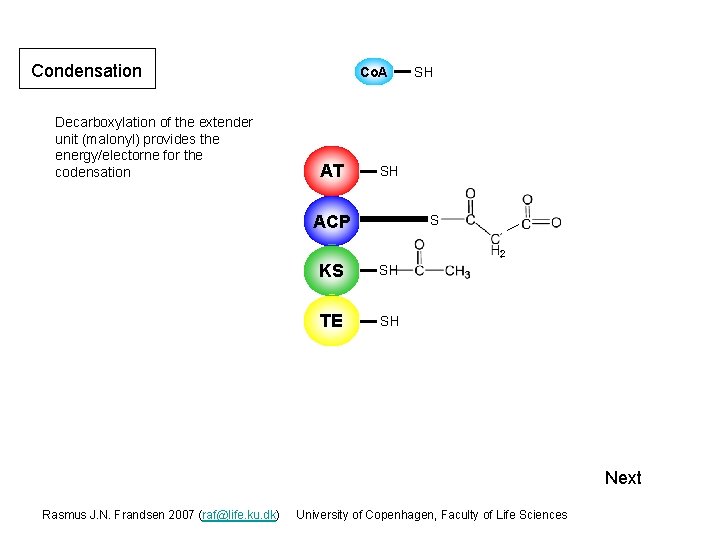
Condensation Decarboxylation of the extender unit (malonyl) provides the energy/electorne for the codensation Co. A AT SH SH ACP S 2 KS S SH TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

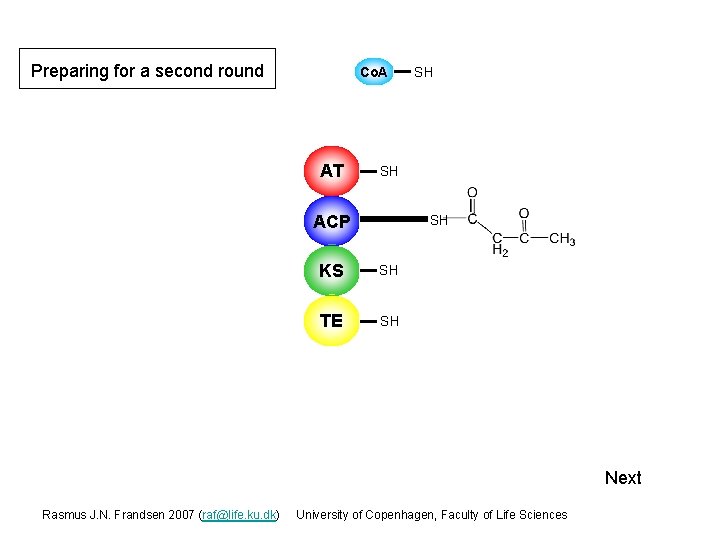
Preparing for a second round Co. A AT SH SH ACP S SH KS SH S TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

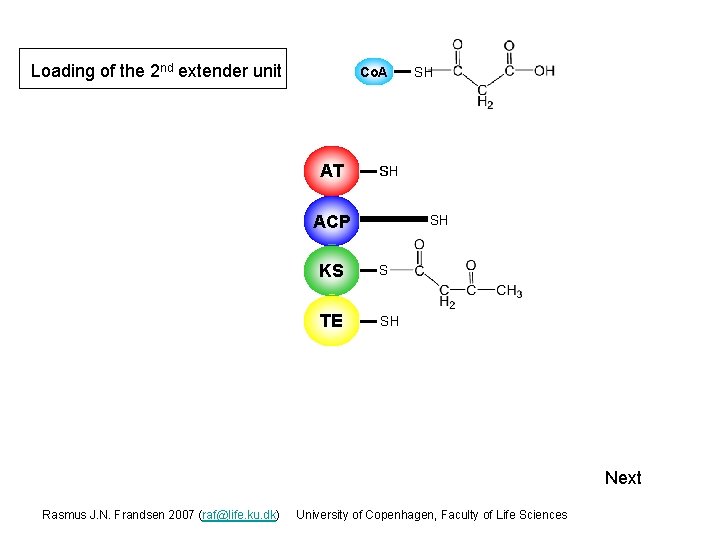
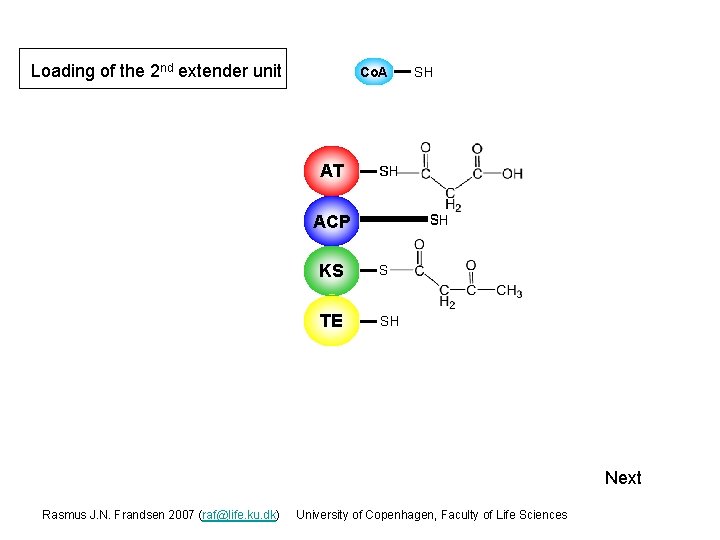
Loading of the 2 nd extender unit Co. A AT S SH SH ACP S SH KS S TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

Loading of the 2 nd extender unit Co. A AT SH SH S ACP S SH KS S TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

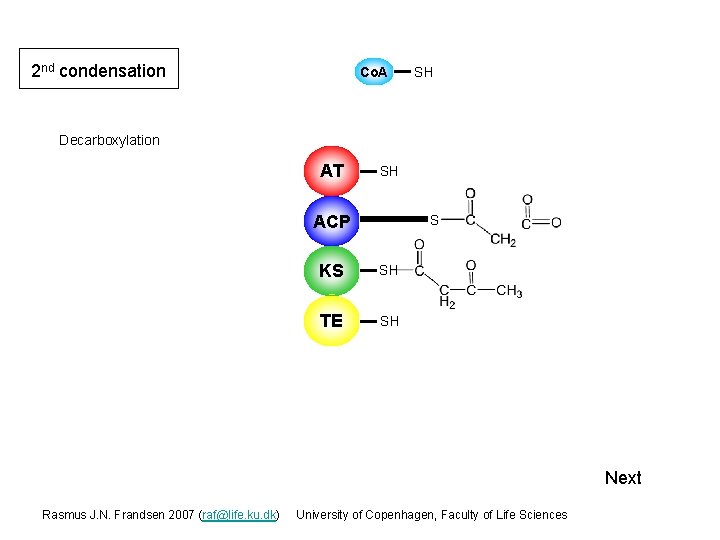
2 nd condensation Co. A SH Decarboxylation AT SH ACP S KS S SH TE SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

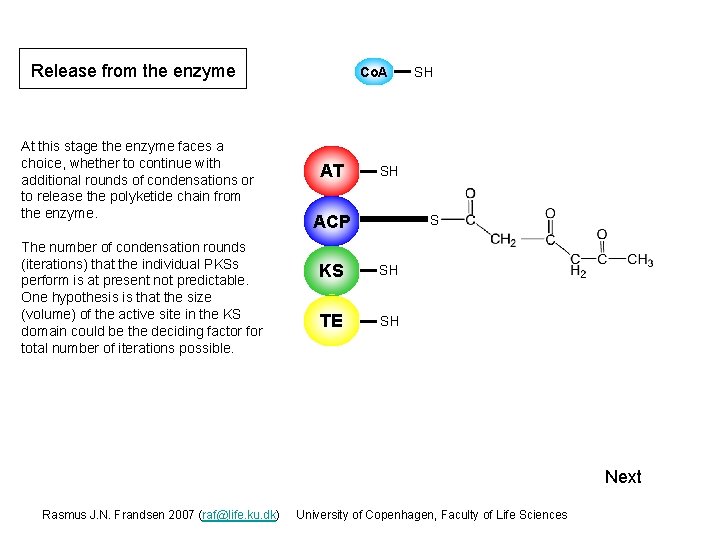
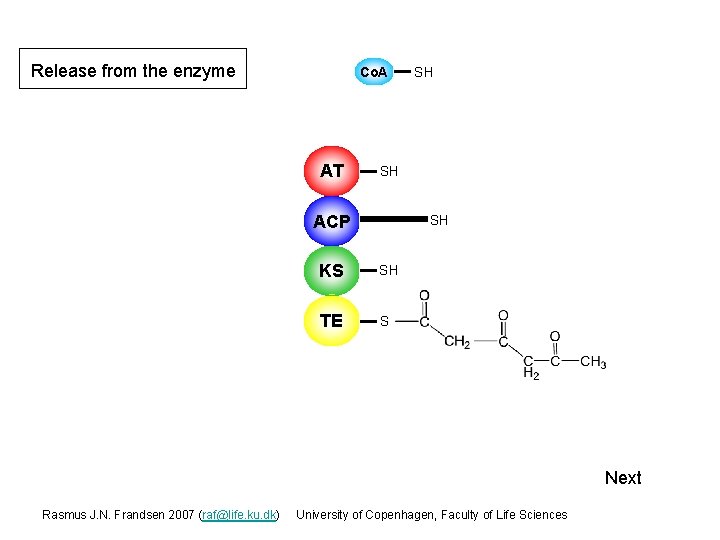
Release from the enzyme At this stage the enzyme faces a choice, whether to continue with additional rounds of condensations or to release the polyketide chain from the enzyme. The number of condensation rounds (iterations) that the individual PKSs perform is at present not predictable. One hypothesis is that the size (volume) of the active site in the KS domain could be the deciding factor for total number of iterations possible. Co. A AT SH SH ACP S KS S SH TE S SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

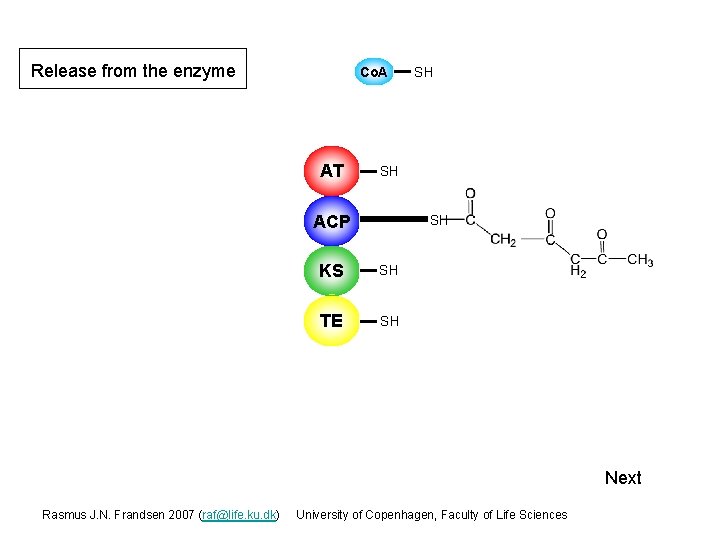
Release from the enzyme Co. A AT SH SH ACP S SH KS S SH TE S SH Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

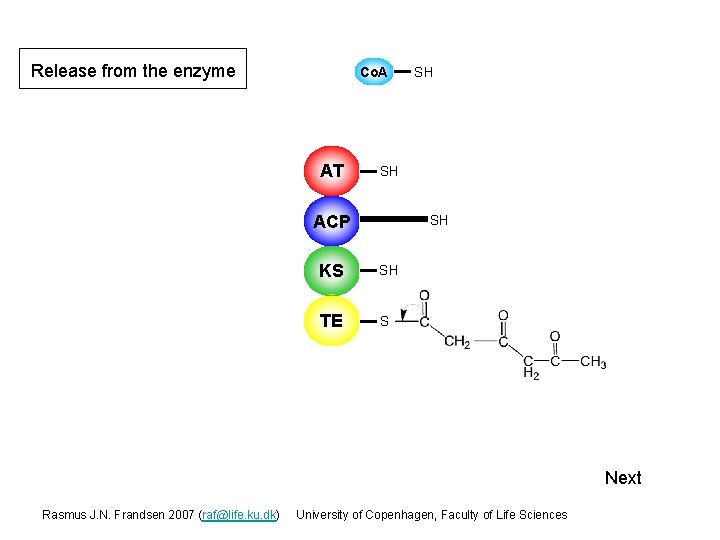
Release from the enzyme Co. A AT SH SH ACP S SH KS S SH TE S Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

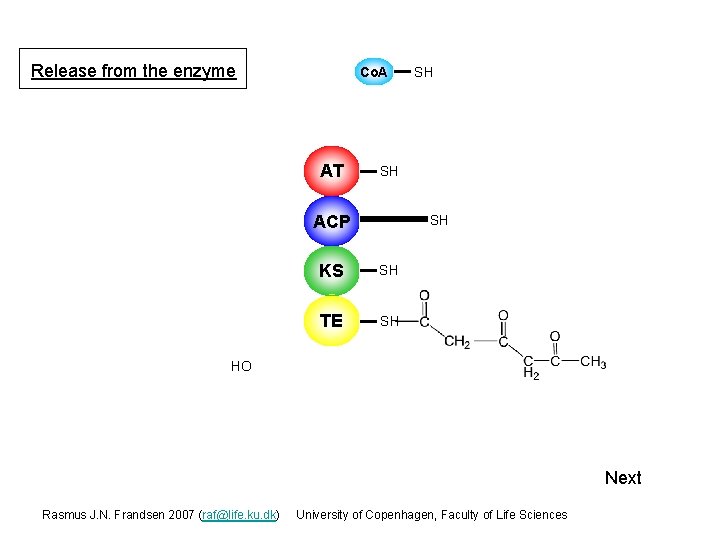
Release from the enzyme Co. A AT SH SH ACP S SH KS S SH TE S Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

Release from the enzyme Co. A AT SH SH ACP S SH KS S SH TE S SH HO Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

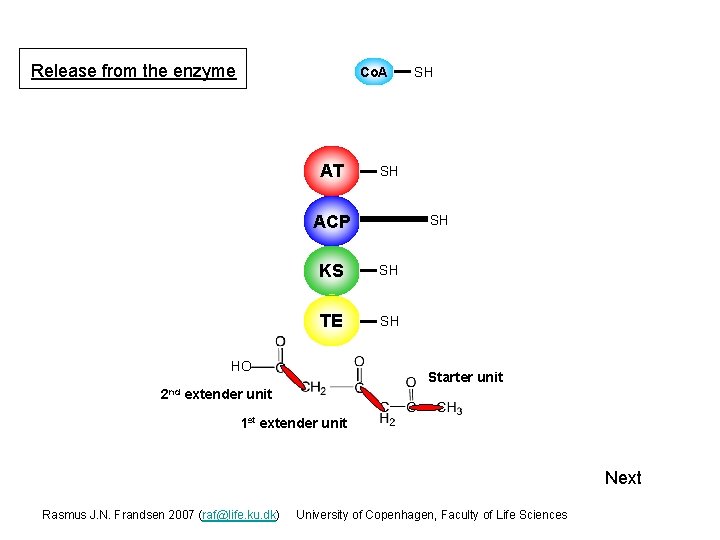
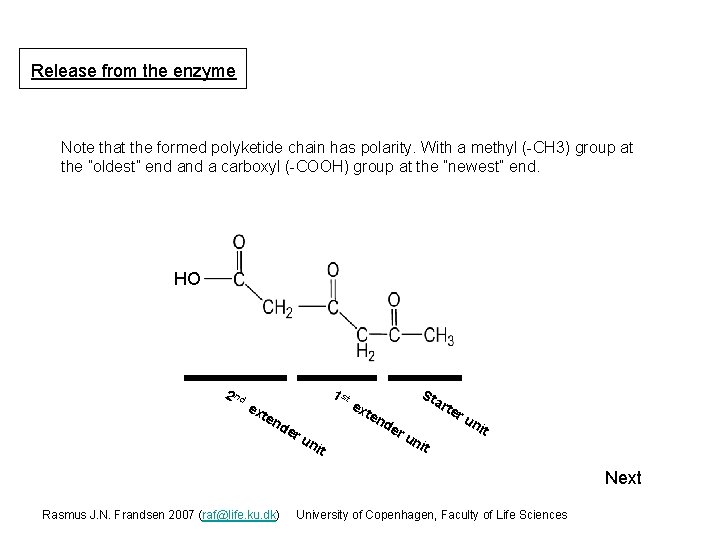
Release from the enzyme Co. A AT SH ACP 2 nd S SH KS S SH TE S SH HO SH Starter unit extender unit 1 st extender unit Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

Release from the enzyme Note that the formed polyketide chain has polarity. With a methyl (-CH 3) group at the ”oldest” end a carboxyl (-COOH) group at the ”newest” end. HO 2 nd 1 st ex ten de ru nit ex ten de St art er ru nit un it Next Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences

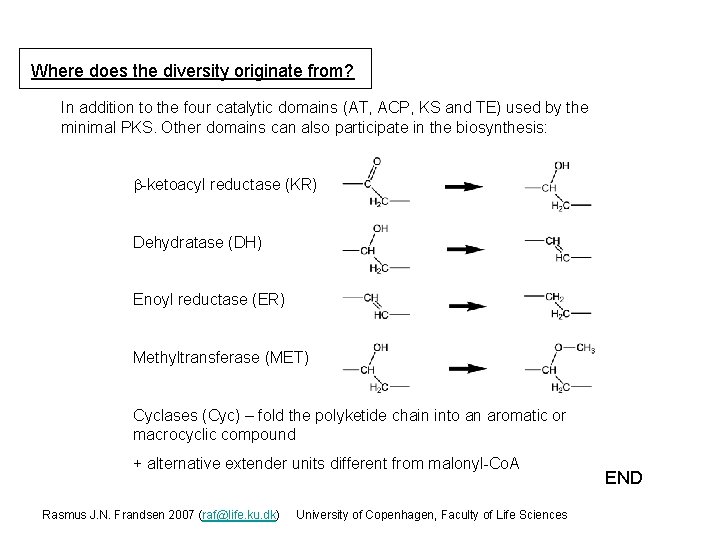
Where does the diversity originate from? In addition to the four catalytic domains (AT, ACP, KS and TE) used by the minimal PKS. Other domains can also participate in the biosynthesis: b-ketoacyl reductase (KR) Dehydratase (DH) Enoyl reductase (ER) Methyltransferase (MET) Cyclases (Cyc) – fold the polyketide chain into an aromatic or macrocyclic compound + alternative extender units different from malonyl-Co. A Rasmus J. N. Frandsen 2007 (raf@life. ku. dk) University of Copenhagen, Faculty of Life Sciences END