Reaction Flask Analysis of the Asymmetric Hydrogenation of


- Slides: 19

Reaction Flask Analysis of the Asymmetric Hydrogenation of Artemisinic Acid Reilly E. Sonstrom, Brooks H. Pate, Department of Chemistry, University of Virginia, Charlottesville, VA Justin Neill, Bright. Spec Inc. , Charlottesville, VA Yuan Yang, B. Frank Gupton, Department of Chemical and Life Science Engineering, Virginia Commonwealth University, Richmond, VA Luca Evangelisti Dipartimento di Chimica G. Ciamician, Università di Bologna ISMS, June 19, 2018

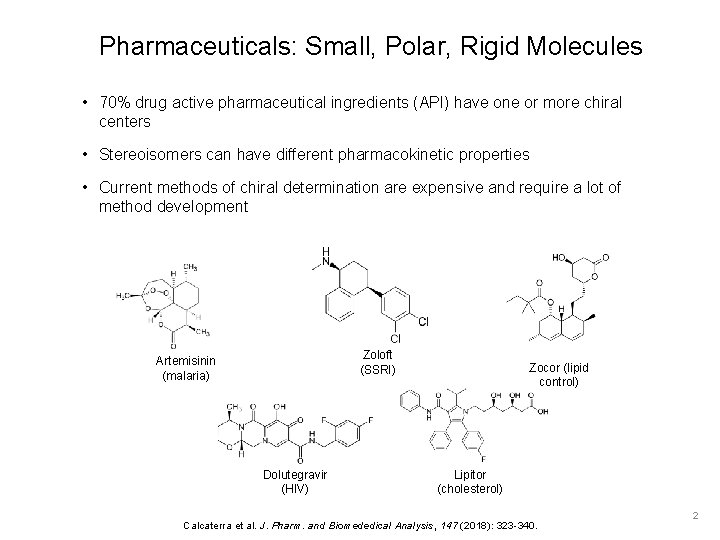
Pharmaceuticals: Small, Polar, Rigid Molecules • 70% drug active pharmaceutical ingredients (API) have one or more chiral centers • Stereoisomers can have different pharmacokinetic properties • Current methods of chiral determination are expensive and require a lot of method development Zoloft (SSRI) Artemisinin (malaria) Dolutegravir (HIV) Zocor (lipid control) Lipitor (cholesterol) Calcaterra et al. J. Pharm. and Biomededical Analysis, 147 (2018): 323 -340. 2

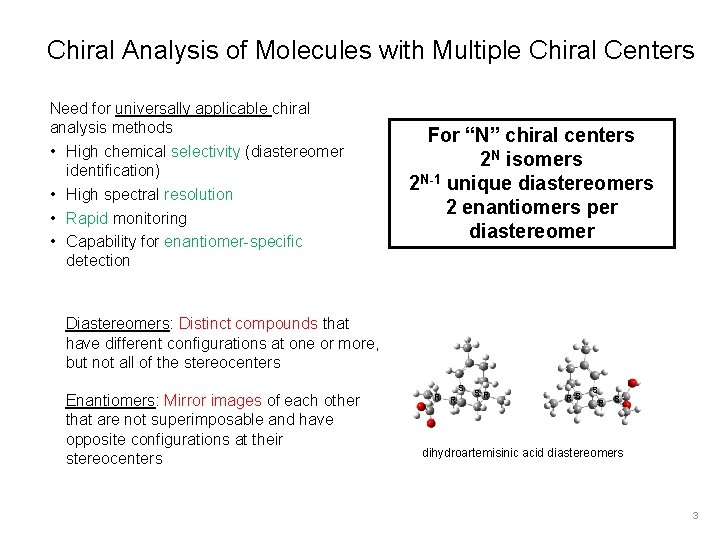
Chiral Analysis of Molecules with Multiple Chiral Centers Need for universally applicable chiral analysis methods • High chemical selectivity (diastereomer identification) • High spectral resolution • Rapid monitoring • Capability for enantiomer-specific detection For “N” chiral centers 2 N isomers 2 N-1 unique diastereomers 2 enantiomers per diastereomer Diastereomers: Distinct compounds that have different configurations at one or more, but not all of the stereocenters Enantiomers: Mirror images of each other that are not superimposable and have opposite configurations at their stereocenters R SR RS S R S dihydroartemisinic acid diastereomers 3

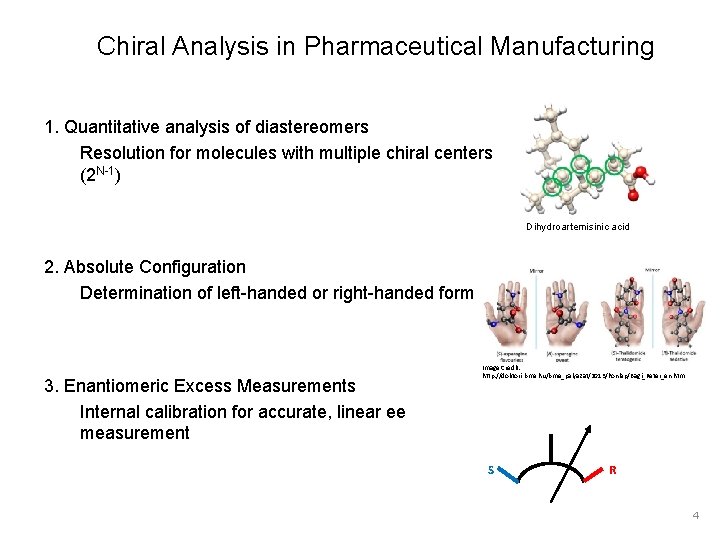
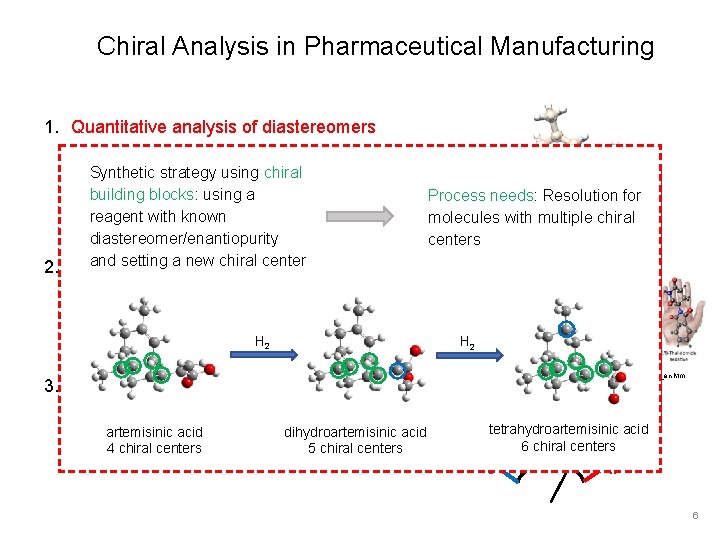
Chiral Analysis in Pharmaceutical Manufacturing 1. Quantitative analysis of diastereomers Resolution for molecules with multiple chiral centers (2 N-1) Dihydroartemisinic acid 2. Absolute Configuration Determination of left-handed or right-handed form 3. Enantiomeric Excess Measurements Internal calibration for accurate, linear ee measurement Image Credit: http: //doktori. bme. hu/bme_palyazat/2013/honlap/Bagi_Peter_en. htm S R 4

Chiral Analysis in Pharmaceutical Manufacturing 1. Quantitative analysis of diastereomers Resolution for molecules with multiple chiral centers (2 N-1) Dihydroartemisinic acid 2. Absolute Configuration Determination of left-handed or right-handed form 3. Enantiomeric Excess Measurements Internal calibration for accurate, linear ee measurement Image Credit: http: //doktori. bme. hu/bme_palyazat/2013/honlap/Bagi_Peter_en. htm S R 5

Chiral Analysis in Pharmaceutical Manufacturing 1. Quantitative analysis of diastereomers Resolution for molecules with multiple chiral centers N-1) strategy using chiral (2 Synthetic building blocks: using a reagent with known diastereomer/enantiopurity and setting a new chiral center 2. Absolute Configuration Process needs: Resolution for molecules with. Dihydroartemisinic multiple chiral Dihydroartemisinic acid centers Determination of left-handed or right-handed form H 2 3. Enantiomeric Excess Measurements Internal calibration for accurate, linear ee artemisinic acid dihydroartemisinic acid measurement 4 chiral centers 5 chiral centers Image Credit: http: //doktori. bme. hu/bme_palyazat/2013/honlap/Bagi_Peter_en. htm tetrahydroartemisinic acid 6 chiral centers S R 6

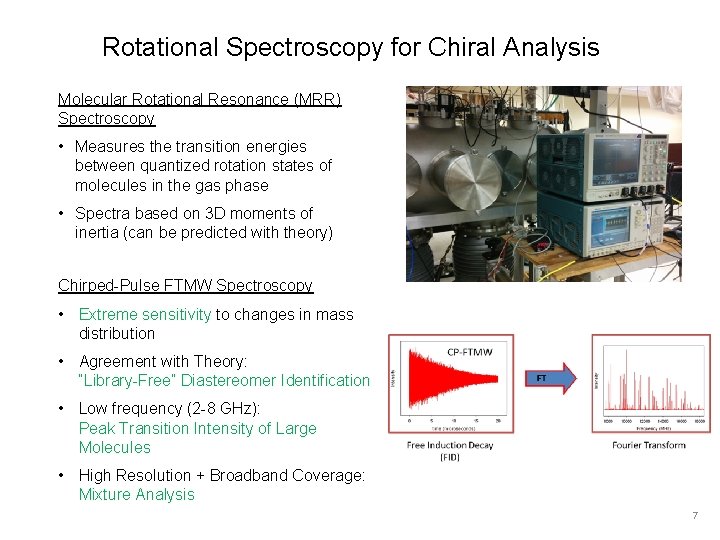
Rotational Spectroscopy for Chiral Analysis Molecular Rotational Resonance (MRR) Spectroscopy • Measures the transition energies between quantized rotation states of molecules in the gas phase • Spectra based on 3 D moments of inertia (can be predicted with theory) Chirped-Pulse FTMW Spectroscopy • Extreme sensitivity to changes in mass distribution • Agreement with Theory: “Library-Free” Diastereomer Identification • Low frequency (2 -8 GHz): Peak Transition Intensity of Large Molecules • High Resolution + Broadband Coverage: Mixture Analysis 7

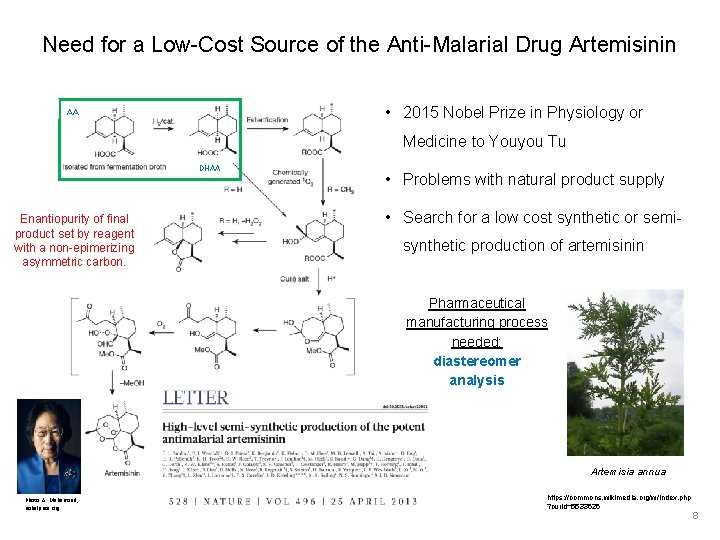
Need for a Low-Cost Source of the Anti-Malarial Drug Artemisinin • 2015 Nobel Prize in Physiology or AA Medicine to Youyou Tu DHAA Enantiopurity of final product set by reagent with a non-epimerizing asymmetric carbon. • Problems with natural product supply • Search for a low cost synthetic or semisynthetic production of artemisinin Pharmaceutical manufacturing process needed: diastereomer analysis Artemisia annua Photo: A. Mahamoud, nobelprize. org https: //commons. wikimedia. org/w/index. php ? curid=5533626 8

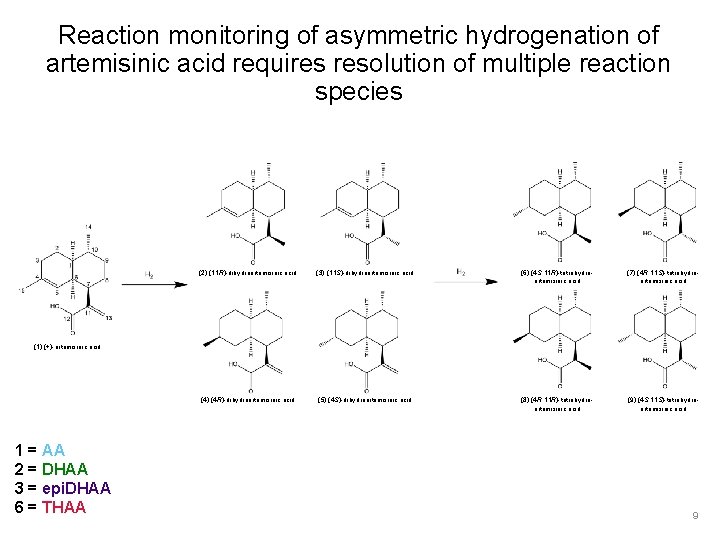
Reaction monitoring of asymmetric hydrogenation of artemisinic acid requires resolution of multiple reaction species (2) (11 R)-dihydroartemisinic acid (3) (11 S)-dihydroartemisinic acid (6) (4 S, 11 R)-tetrahydroartemisinic acid (7) (4 R, 11 S)-tetrahydroartemisinic acid (4) (4 R)-dihydroartemisinic acid (5) (4 S)-dihydroartemisinic acid (8) (4 R, 11 R)-tetrahydroartemisinic acid (9) (4 S, 11 S)-tetrahydroartemisinic acid (1) (+)-artemisinic acid 1 = AA 2 = DHAA 3 = epi. DHAA 6 = THAA 9

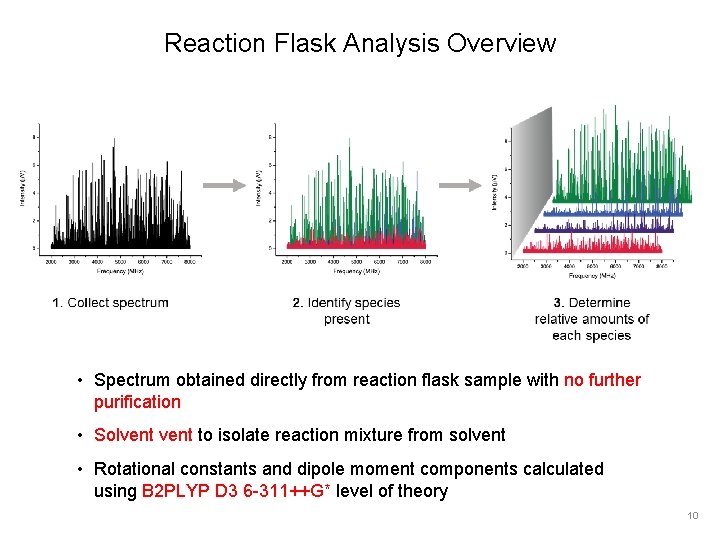
Reaction Flask Analysis Overview • Spectrum obtained directly from reaction flask sample with no further purification • Solvent to isolate reaction mixture from solvent • Rotational constants and dipole moment components calculated using B 2 PLYP D 3 6 -311++G* level of theory 10

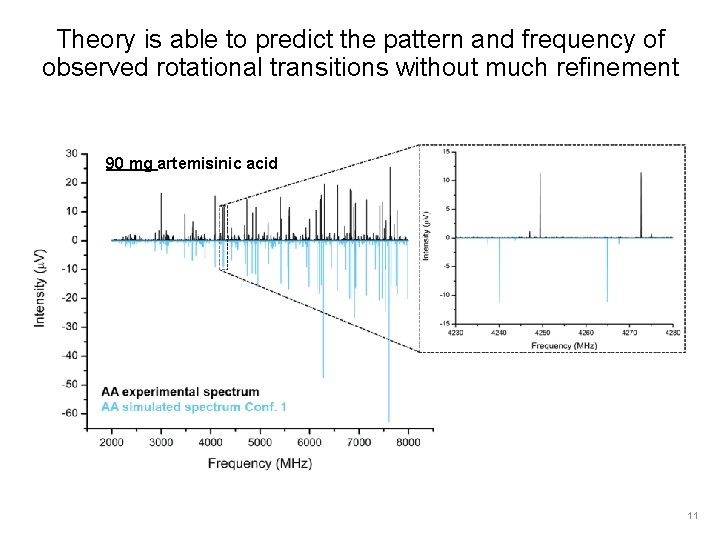
Theory is able to predict the pattern and frequency of observed rotational transitions without much refinement 90 mg artemisinic acid 11

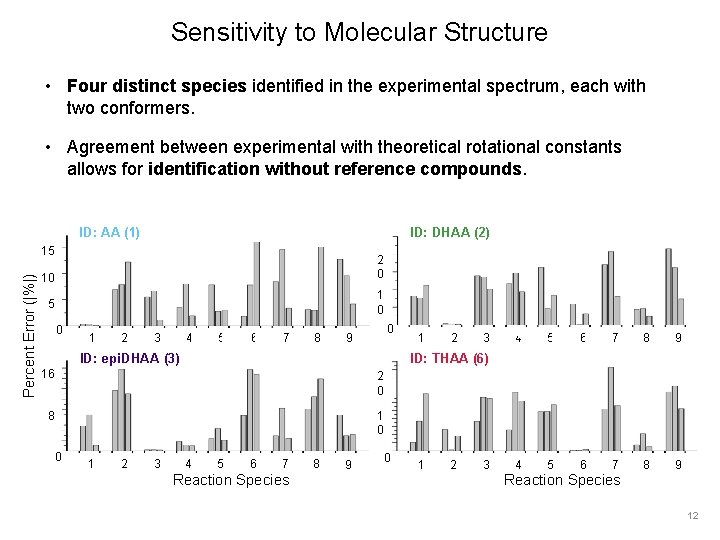
Sensitivity to Molecular Structure • Four distinct species identified in the experimental spectrum, each with two conformers. • Agreement between experimental with theoretical rotational constants allows for identification without reference compounds. ID: AA (1) ID: DHAA (2) Percent Error (|%|) 15 10 2 0 5 1 0 0 1 2 3 4 5 6 7 8 00 9 ID: epi. DHAA (3) 2 0 8 1 0 1 2 3 4 5 6 7 8 9 ID: THAA (6) 16 0 1 4 5 6 7 Reaction Species 8 9 0 1 2 3 Reaction Species 12

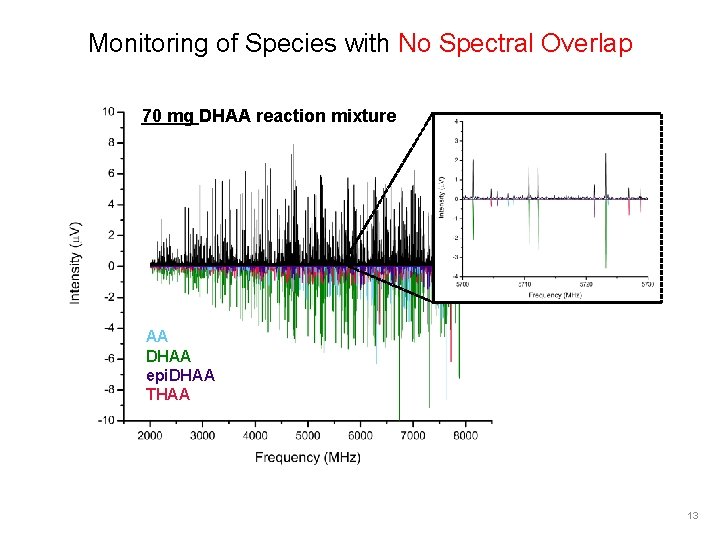
Monitoring of Species with No Spectral Overlap 70 mg DHAA reaction mixture AA DHAA epi. DHAA THAA 13

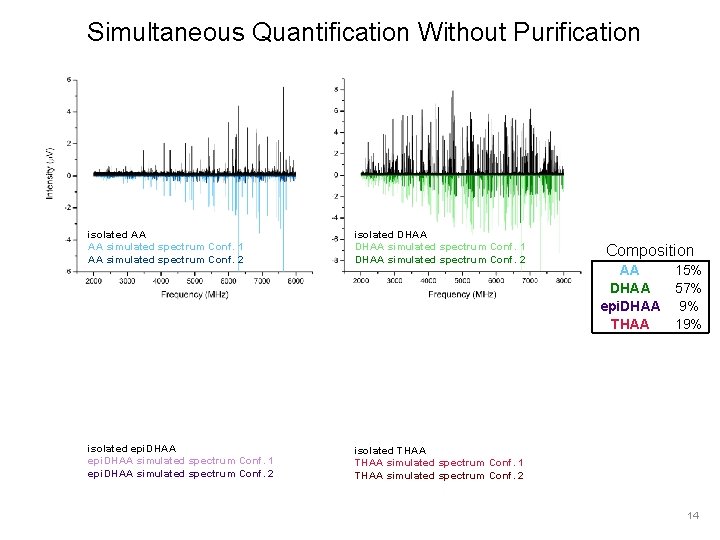
Simultaneous Quantification Without Purification isolated AA AA simulated spectrum Conf. 1 AA simulated spectrum Conf. 2 isolated DHAA simulated spectrum Conf. 1 DHAA simulated spectrum Conf. 2 isolated epi. DHAA simulated spectrum Conf. 1 epi. DHAA simulated spectrum Conf. 2 isolated THAA simulated spectrum Conf. 1 THAA simulated spectrum Conf. 2 Composition AA DHAA epi. DHAA THAA 15% 57% 9% 14

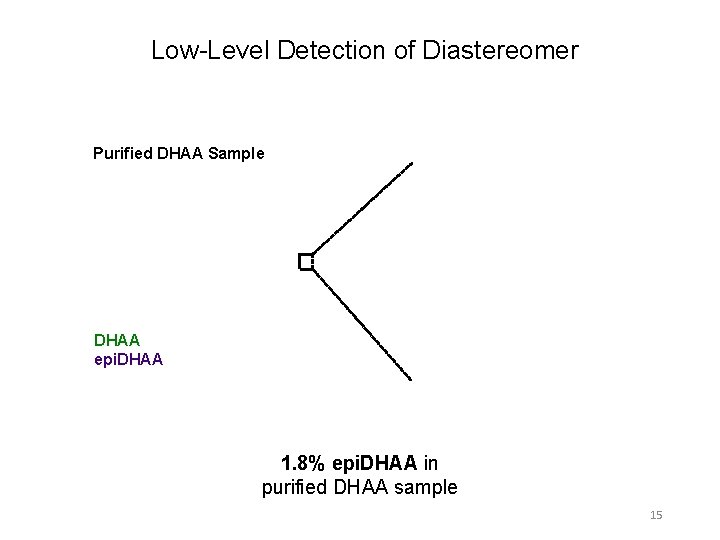
Low-Level Detection of Diastereomer Purified DHAA Sample DHAA epi. DHAA 1. 8% epi. DHAA in purified DHAA sample 15

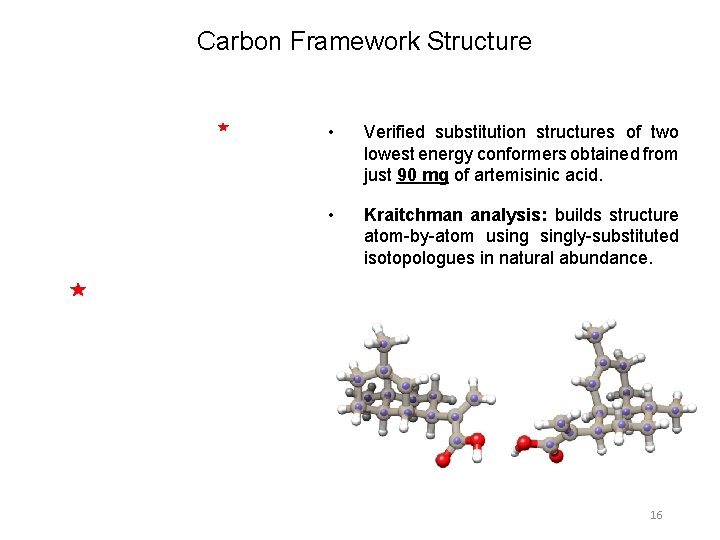
Carbon Framework Structure • Verified substitution structures of two lowest energy conformers obtained from just 90 mg of artemisinic acid. • Kraitchman analysis: builds structure atom-by-atom usingly-substituted isotopologues in natural abundance. 16

Conclusions • MRR has extreme sensitivity to changes in mass distribution. • Agreement between theoretical and experimental rotational constants allows for identification without reference compounds. • High sensitivity and spectral resolution allows us to observe all species with no spectral overlap from a 70 mg reaction flask sample • Good sensitivity on molecules of this size is noteworthy (fairly routine measurement of molecules <300 amu) 17

Acknowledgements Brooks H. Pate Luca Evangelisti Bright. Spec: Justin Neill, Matt Muckle VCU Medicines for All Institute: B. Frank Gupton, Yuan Yang, Thomas Roper, Jo-Ann Jee Pate Lab Group: Martin Holdren, Kevin Meyer, Taylor Smart, Channing West 18

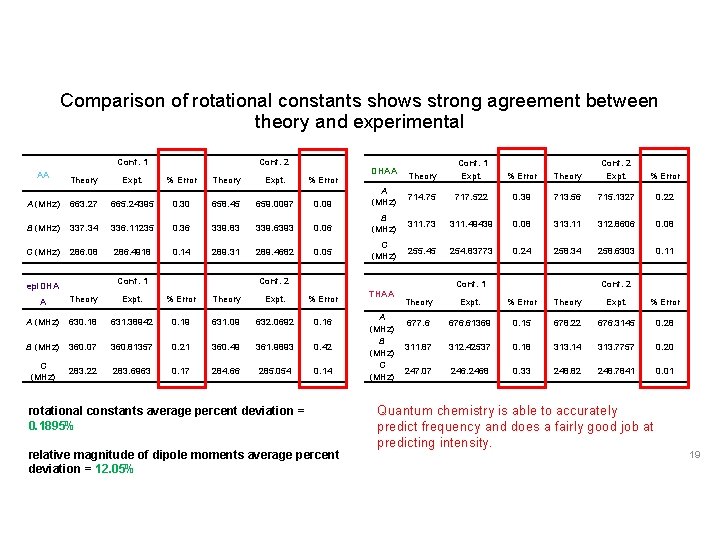
Comparison of rotational constants shows strong agreement between theory and experimental Conf. 1 AA Conf. 2 DHAA Theory Conf. 1 Expt. % Error Theory Conf. 2 Expt. % Error Theory Expt. % Error A (MHz) 663. 27 665. 24395 0. 30 658. 45 659. 0097 0. 09 A (MHz) 714. 75 717. 522 0. 39 713. 56 715. 1327 0. 22 B (MHz) 337. 34 336. 11235 0. 36 339. 83 339. 6393 0. 06 B (MHz) 311. 73 311. 49439 0. 08 313. 11 312. 8606 0. 08 C (MHz) 286. 08 286. 4918 0. 14 289. 31 289. 4682 0. 05 C (MHz) 255. 45 254. 83773 0. 24 258. 34 258. 6303 0. 11 THAA Conf. 1 epi. DHA Conf. 2 Conf. 1 A Theory Expt. % Error A (MHz) 630. 18 631. 38942 0. 19 631. 09 632. 0692 0. 16 B (MHz) 360. 07 360. 81357 0. 21 360. 49 361. 9893 0. 42 C (MHz) 283. 22 283. 6963 0. 17 284. 66 285. 054 0. 14 rotational constants average percent deviation = 0. 1895% relative magnitude of dipole moments average percent deviation = 12. 05% A (MHz) B (MHz) Conf. 2 Theory Expt. % Error 677. 6 676. 61369 0. 15 678. 22 676. 3145 0. 28 311. 87 312. 42537 0. 18 313. 14 313. 7757 0. 20 247. 07 2468 0. 33 248. 82 248. 7841 0. 01 Quantum chemistry is able to accurately predict frequency and does a fairly good job at predicting intensity. 19