Reaction Equilibrium I Heterogenous vs Homogenous Reactions A

Reaction Equilibrium
I. Heterogenous vs. Homogenous Reactions A. Homogeneous reaction – all reactants and products are in one phase • • • All in the gaseous phase, or All in solution (aqueous) H 2 O (g) + CO (g) H 2 (g) + CO 2 (g) B. Heterogeneous reaction – reactants in two phases • Zn(s) + 2 HCl(aq) H 2(g) + Zn. Cl 2(aq)
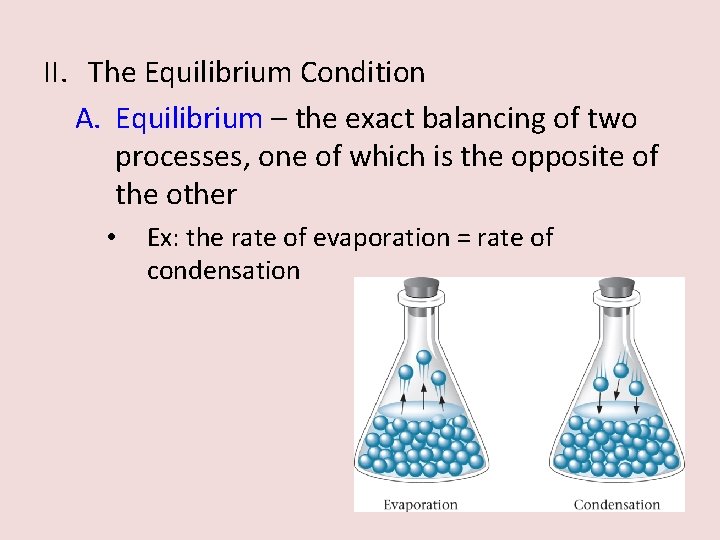
II. The Equilibrium Condition A. Equilibrium – the exact balancing of two processes, one of which is the opposite of the other • Ex: the rate of evaporation = rate of condensation
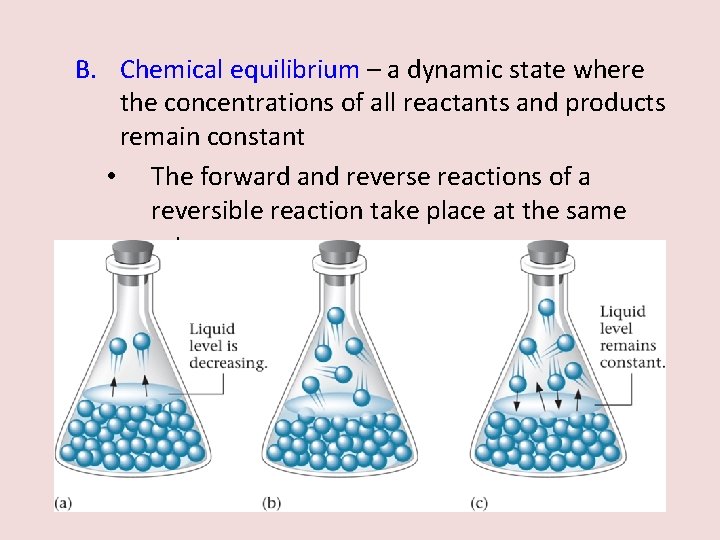
B. Chemical equilibrium – a dynamic state where the concentrations of all reactants and products remain constant • The forward and reverse reactions of a reversible reaction take place at the same rate
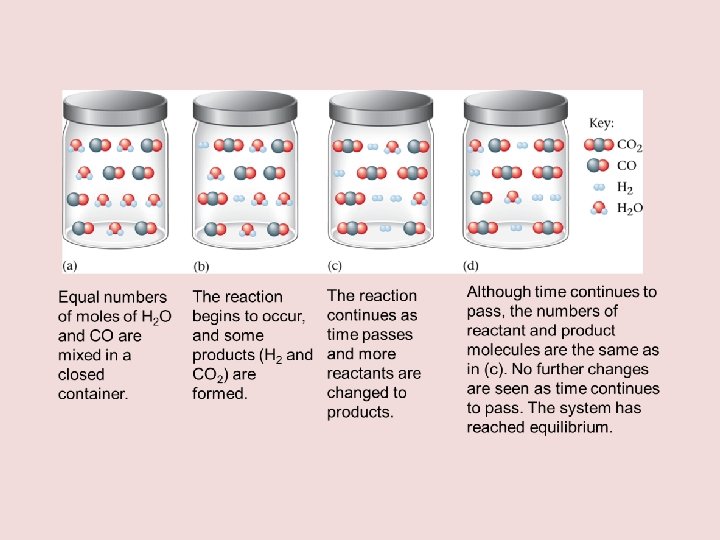
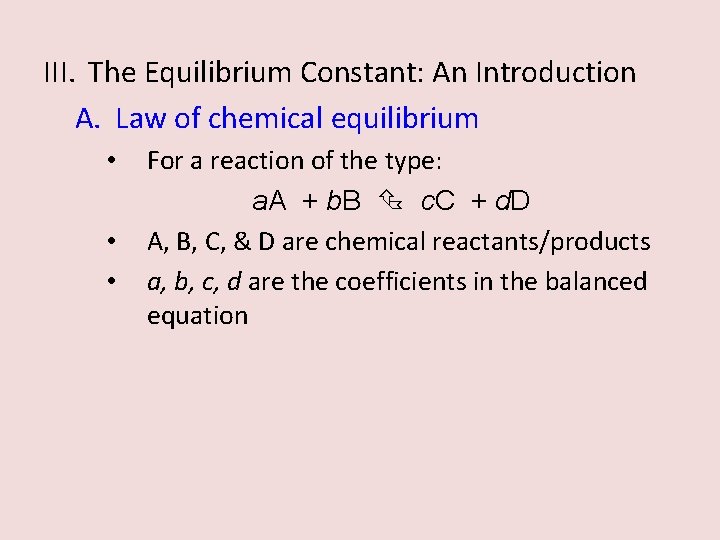
III. The Equilibrium Constant: An Introduction A. Law of chemical equilibrium • • • For a reaction of the type: a. A + b. B c. C + d. D A, B, C, & D are chemical reactants/products a, b, c, d are the coefficients in the balanced equation
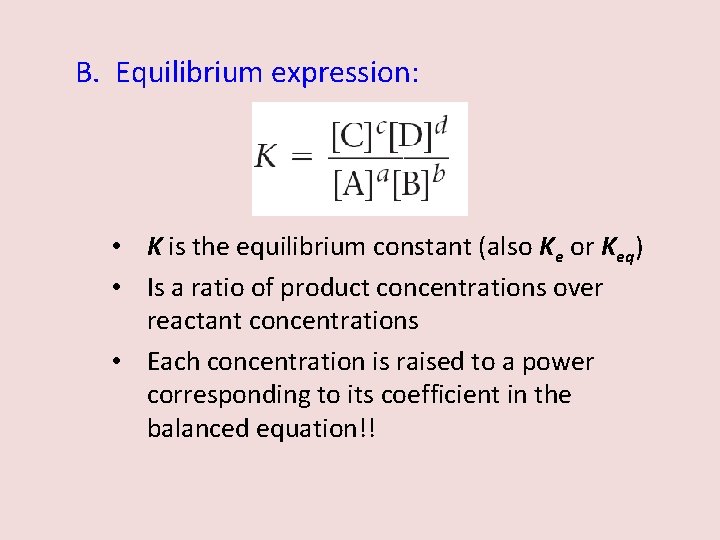
B. Equilibrium expression: • K is the equilibrium constant (also Ke or Keq) • Is a ratio of product concentrations over reactant concentrations • Each concentration is raised to a power corresponding to its coefficient in the balanced equation!!
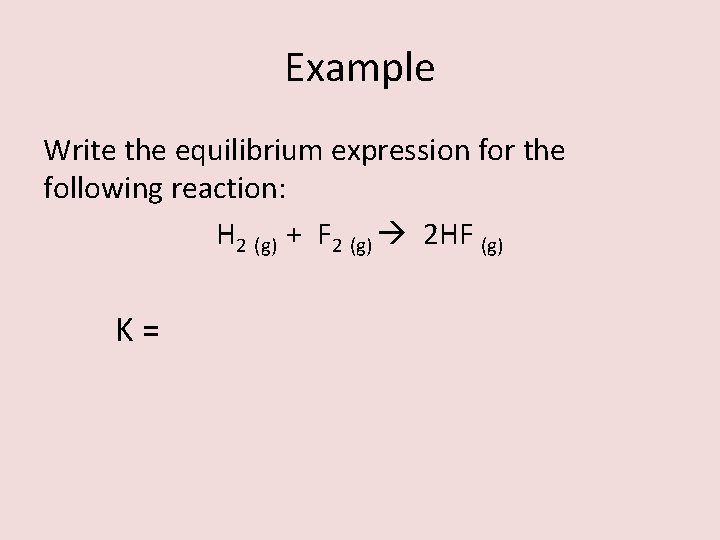
Example Write the equilibrium expression for the following reaction: H 2 (g) + F 2 (g) 2 HF (g) K=
![Example Using the equilibrium expression we wrote in the previous slide if: [H 2] Example Using the equilibrium expression we wrote in the previous slide if: [H 2]](http://slidetodoc.com/presentation_image_h/361f97e1356c721dbf070723c9d50bc5/image-9.jpg)
Example Using the equilibrium expression we wrote in the previous slide if: [H 2] = 1. 000 M [F 2] = 2. 000 M K= [HF] = 3. 000 M

IV. Heterogeneous Equilibria A. an equilibrium system where the products and reactants are not all in the same state B. When solving for K, do not include the concentrations of solids and liquids • The concentrations of solids and liquids cannot change, so are not included in K C. Only include the gases and solutions (aqueous) when solving for K.
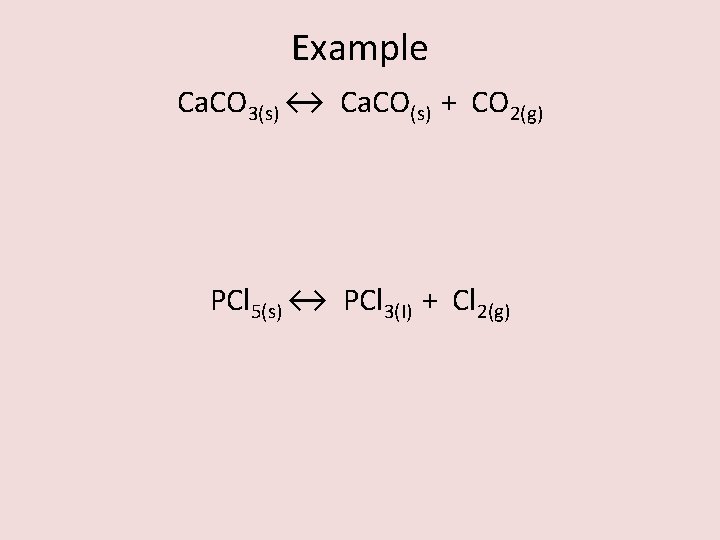
Example Ca. CO 3(s) ↔ Ca. CO(s) + CO 2(g) PCl 5(s) ↔ PCl 3(l) + Cl 2(g)
- Slides: 11