Reaction Equations Chemical Equations An expression in which





- Slides: 15

Reaction Equations

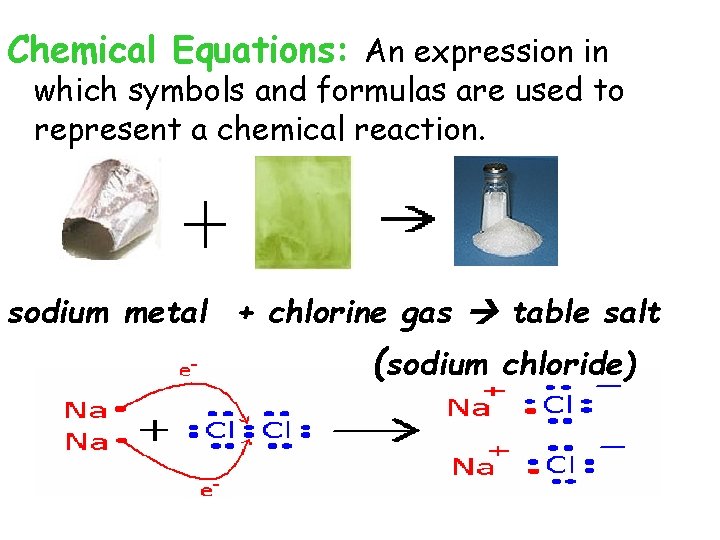
Chemical Equations: An expression in which symbols and formulas are used to represent a chemical reaction. sodium metal + chlorine gas table salt (sodium chloride)

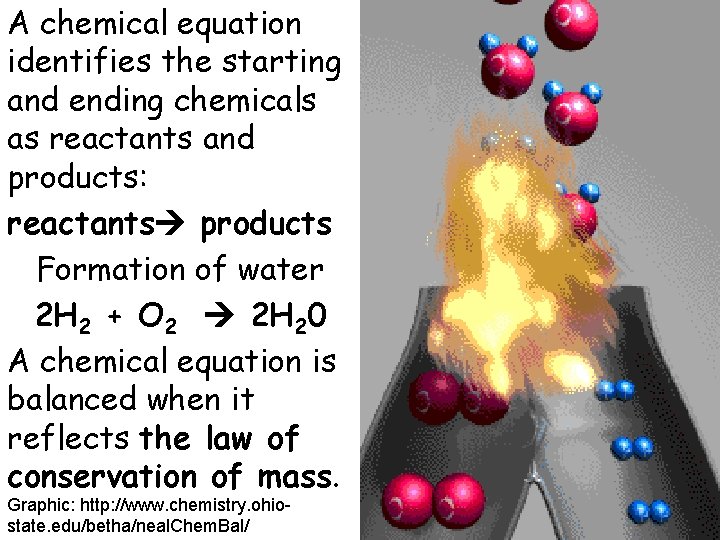
A chemical equation identifies the starting and ending chemicals as reactants and products: reactants products Formation of water 2 H 2 + O 2 2 H 20 A chemical equation is balanced when it reflects the law of conservation of mass. Graphic: http: //www. chemistry. ohiostate. edu/betha/neal. Chem. Bal/

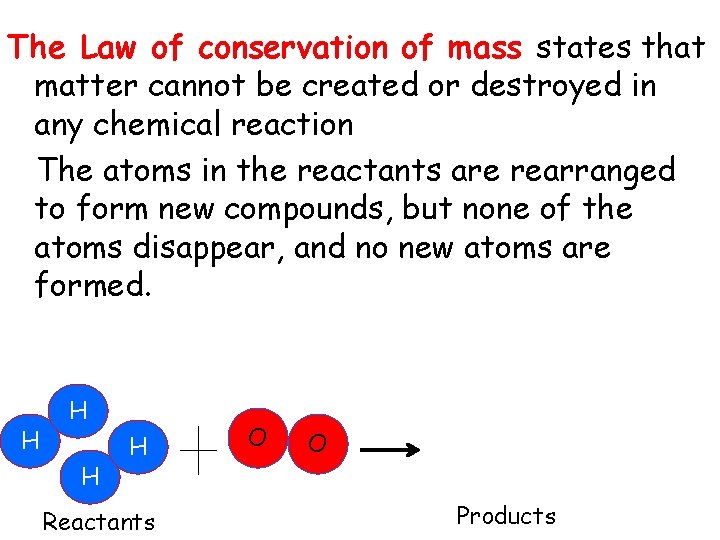
The Law of conservation of mass states that matter cannot be created or destroyed in any chemical reaction The atoms in the reactants are rearranged to form new compounds, but none of the atoms disappear, and no new atoms are formed. H H Reactants O O Products

Remember that atoms don’t change in a chemical reaction. • The number and kinds of atoms present in the reactants of a chemical reaction are the same as those present in the products. When stated this way, it becomes the law of conservation of atoms.

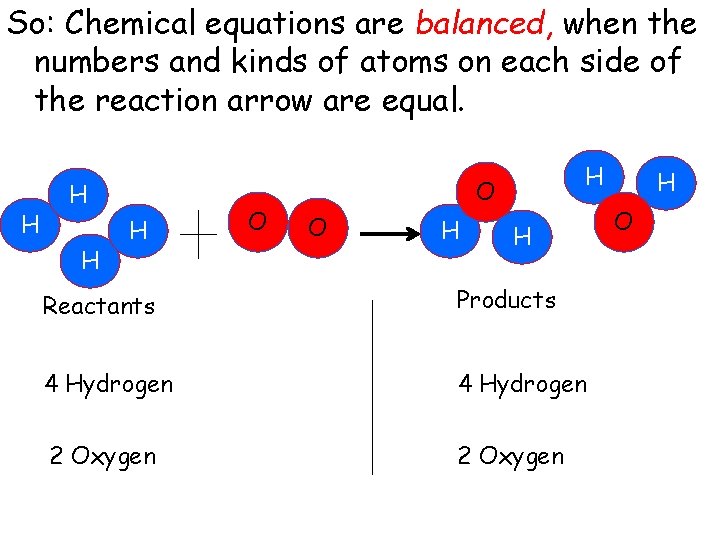
So: Chemical equations are balanced, when the numbers and kinds of atoms on each side of the reaction arrow are equal. H H O H O O H H Reactants Products 4 Hydrogen 2 Oxygen H O

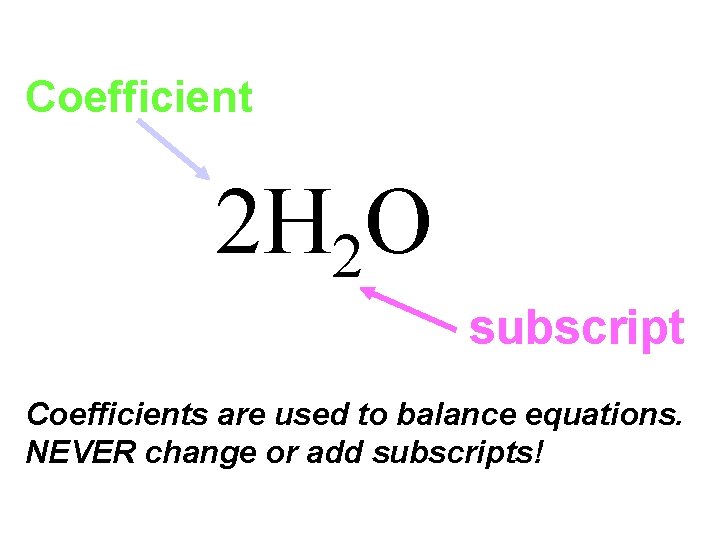
Coefficients, the numbers placed in front of formulas to balance equations. They indicate the number of particles present in the reaction. If a number is not present it is understood that 1 is the coefficient. 2 H 2 + O 2 2 H 2 O

Coefficient 2 H 2 O subscript Coefficients are used to balance equations. NEVER change or add subscripts!

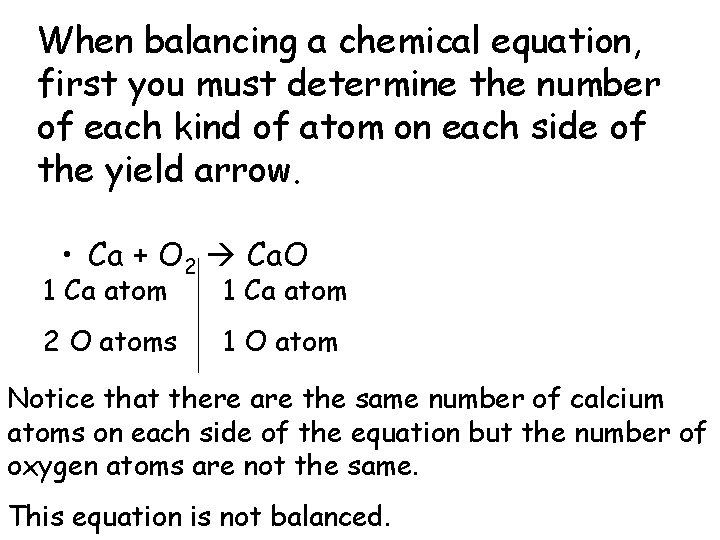
When balancing a chemical equation, first you must determine the number of each kind of atom on each side of the yield arrow. • Ca + O 2 Ca. O 1 Ca atom 2 O atoms 1 O atom Notice that there are the same number of calcium atoms on each side of the equation but the number of oxygen atoms are not the same. This equation is not balanced.

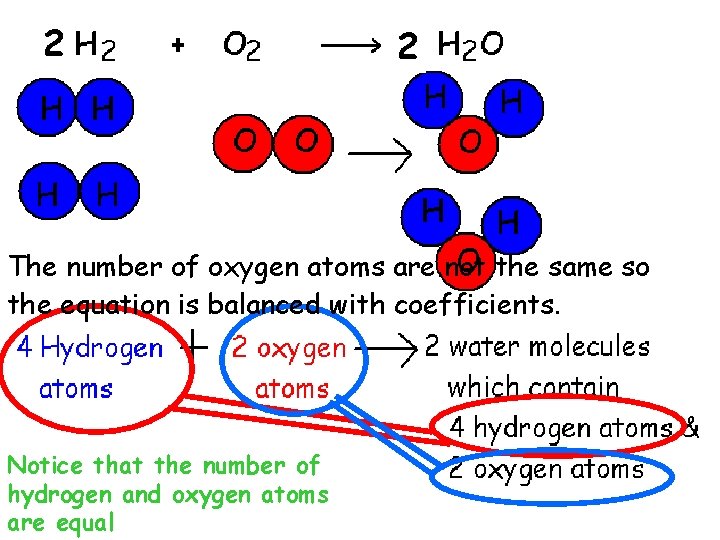
2 2 The number of oxygen atoms are not the same so the equation is balanced with coefficients. Notice that the number of hydrogen and oxygen atoms are equal

Balancing Chemical Equations The following five steps can be used as a guide to balance chemical equations. Balance this chemical reaction. Sulfuric Acid reacts with sodium hydroxide to yield sodium sulfate and water Step 1: Write an unbalanced equation, using correct formulas for all reactants and products. H 2 SO 4 + Na. OH Na 2 SO 4 + H 2 O

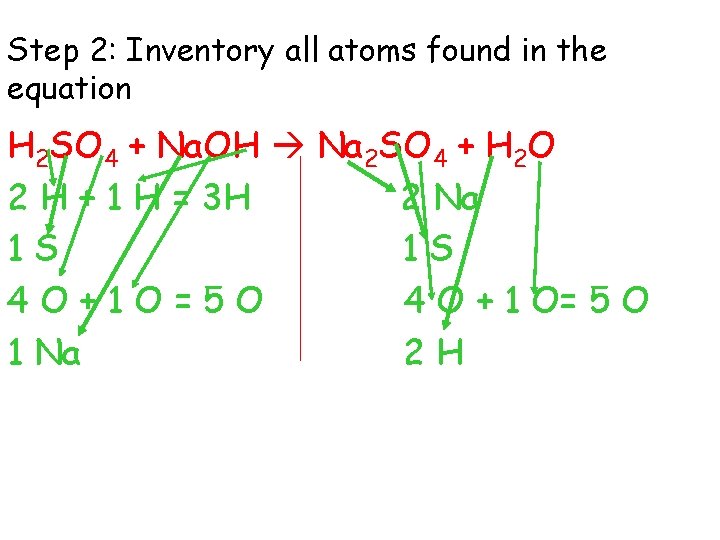
Step 2: Inventory all atoms found in the equation H 2 SO 4 + Na. OH Na 2 SO 4 + H 2 O 2 H + 1 H = 3 H 2 Na 1 S 1 S 4 O+1 O=5 O 4 O + 1 O= 5 O 1 Na 2 H

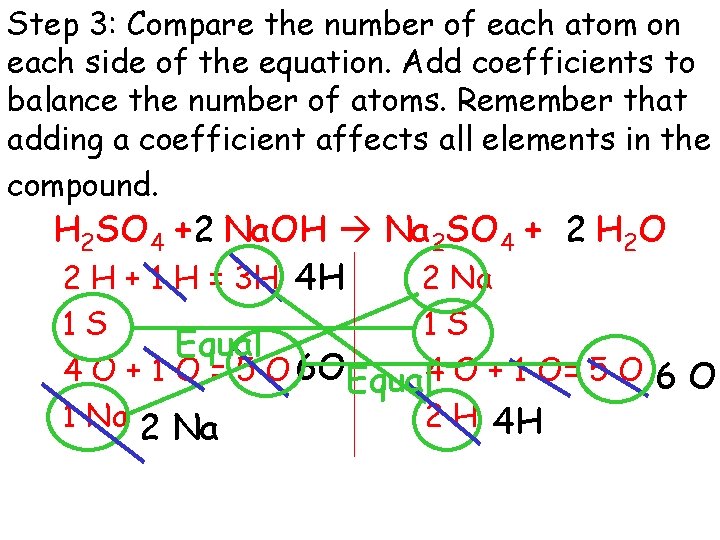
Step 3: Compare the number of each atom on each side of the equation. Add coefficients to balance the number of atoms. Remember that adding a coefficient affects all elements in the compound. H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O 2 H + 1 H = 3 H 4 H 2 Na 1 S 1 S Equal 4 O + 1 O = 5 O 6 OEqual 4 O + 1 O= 5 O 6 O 1 Na 2 H 4 H

Step 4: Check the equation to make sure the numbers and kinds of atoms on both sides of the equation are same. H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O 2 H + 2 H = 4 H 2 Na 1 S 1 S 4 O+2 O=6 O 4 O + 2 O= 6 O 2 Na 4 H

Step 5: Make sure the coefficients are reduced to their lowest wholenumber value (ok here). H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O 1: 2: 1: 2