Reaction Endothermic Balancing Important Rate or Equations Laws
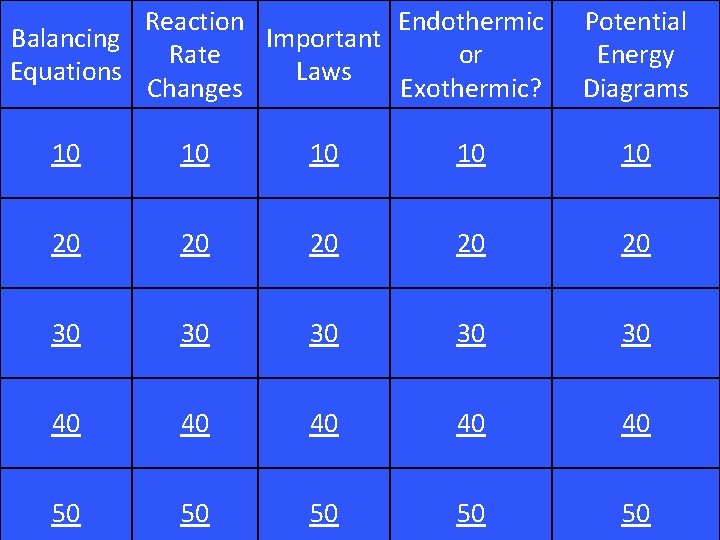
Reaction Endothermic Balancing Important Rate or Equations Laws Changes Exothermic? Potential Energy Diagrams 10 10 10 20 20 20 30 30 30 40 40 40 50 50 50
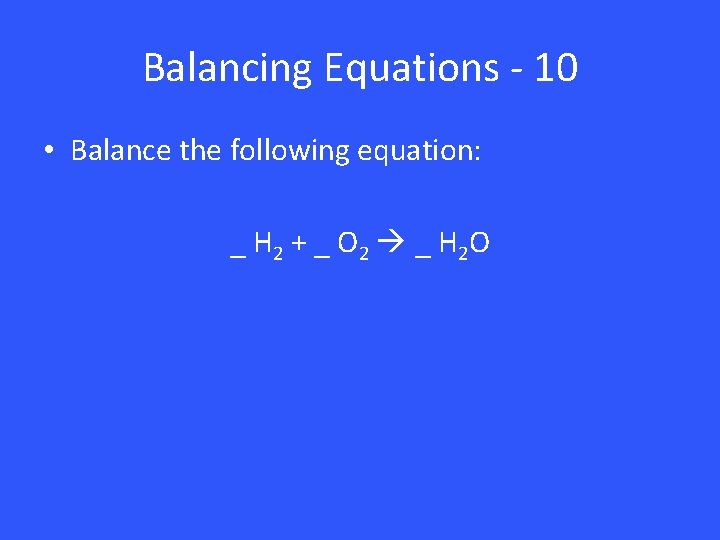
Balancing Equations - 10 • Balance the following equation: _ H 2 + _ O 2 _ H 2 O

Balancing Equations - 10 2 H 2 + 1 O 2 2 H 2 O
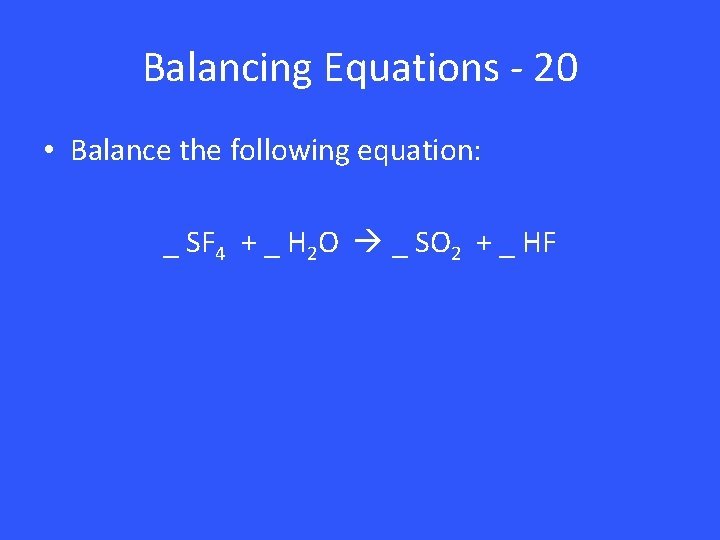
Balancing Equations - 20 • Balance the following equation: _ SF 4 + _ H 2 O _ SO 2 + _ HF

Balancing Equations - 20 1 SF 4 + 2 H 2 O 1 SO 2 + 4 HF
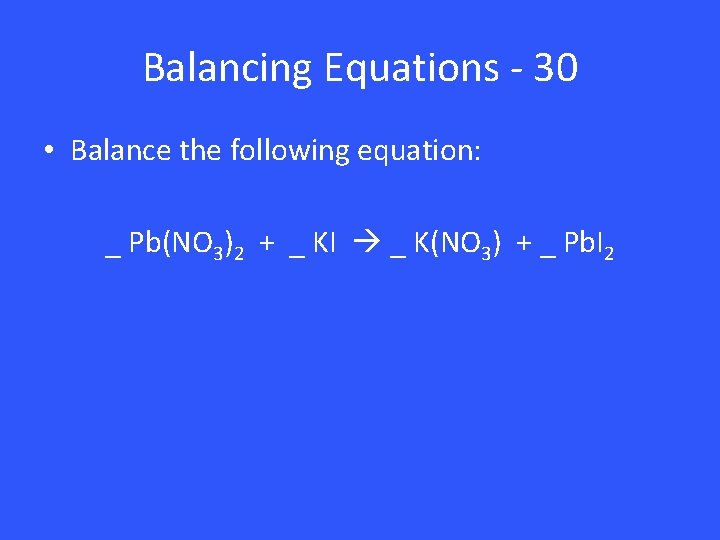
Balancing Equations - 30 • Balance the following equation: _ Pb(NO 3)2 + _ KI _ K(NO 3) + _ Pb. I 2

Balancing Equations – 30 1 Pb(NO 3)2 + 2 KI 2 K(NO 3) + 1 Pb. I 2
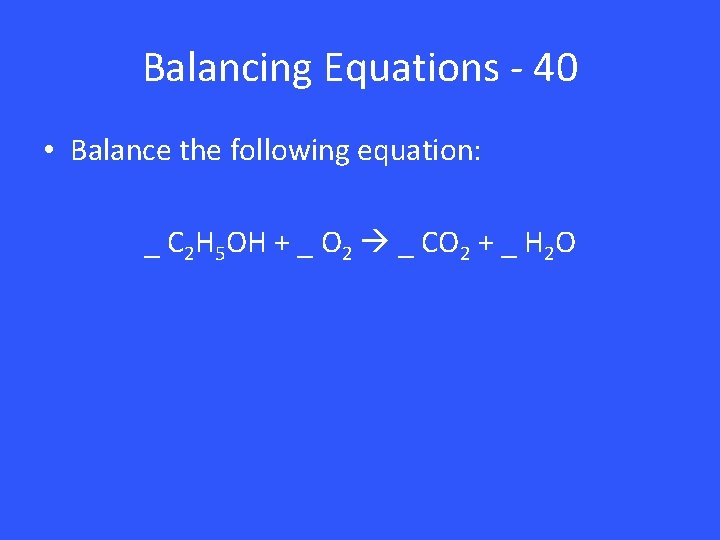
Balancing Equations - 40 • Balance the following equation: _ C 2 H 5 OH + _ O 2 _ CO 2 + _ H 2 O

Balancing Equations – 40 1 C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O
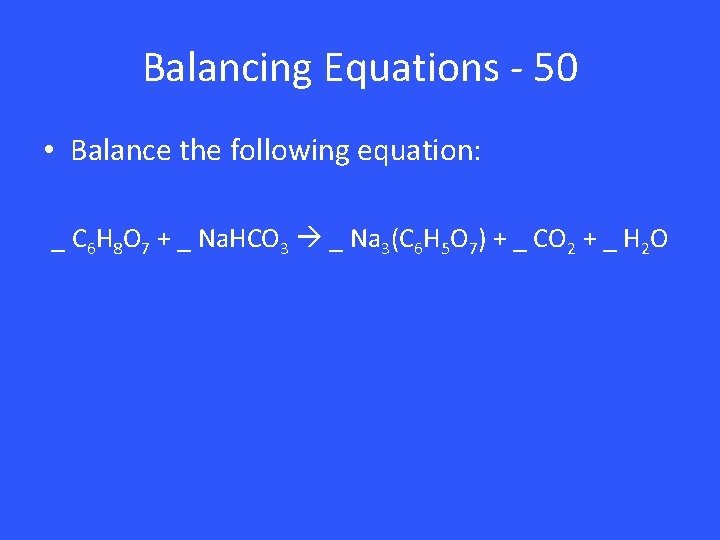
Balancing Equations - 50 • Balance the following equation: _ C 6 H 8 O 7 + _ Na. HCO 3 _ Na 3(C 6 H 5 O 7) + _ CO 2 + _ H 2 O

Balancing Equations – 50 1 C 6 H 8 O 7 + 3 Na. HCO 3 1 Na 3(C 6 H 5 O 7) + 3 CO 2 + 3 H 2 O

Reaction Rate Changes - 10 • What happens to reaction rate if concentration in increased AND why does this happen?

Reaction Rate Changes – 10 • Reaction rate increases, more particles = more collisions, and more collisions is higher chance of good collision

Reaction Rate Changes - 20 • What would happen to reaction rate if surface area was decreased AND why does this happen?

Reaction Rate Changes – 20 • Reaction rate decreases, less surface area means less exposure for atoms on the inside of a structure, which means less collisions

Reaction Rate Changes - 30 • If you increased the temperature of a chemical reaction, would the reaction rate increase or decrease AND why?

Reaction Rate Changes – 30 • Reaction rate would increase because molecules will have a higher kinetic energy, which means higher energy collisions (good collisions)

Reaction Rate Changes - 40 • What does a catalyst do a chemical reaction? How does it achieve that effect?

Reaction Rate Changes – 40 Increases reaction rate by aligning chemicals in the correct orientation to have a good collision

Reaction Rate Changes - 50 • Mr. Leung wanted to observe a chemical reaction, but it was happening too quickly. What are all of the ways Mr. Leung could slow down the reaction rate to observe it better?

Reaction Rate Changes – 50 • Decrease concentration by removing some reactants • Decrease surface area of chemicals by lumping them together • Decrease temperature by adding ice or turning heat source off • Remove catalyst (If there was one)

Important Laws - 10 • What is the most important law of chemistry?

Important Laws – 10 • The Law of Conservation of Matter

Important Laws - 20 • How does the Law of Conservation of Matter apply to balanced equations?

Important Laws – 20 • Need to have same amount of atoms on both sides (If you start with 8 iron atoms, you need to end with 8 iron atoms)

Important Laws - 30 • What needs to happen for a chemical reaction to occur?

Important Laws – 30 • Reactants need to collide

Important Laws - 40 • We know that a chemical reaction needs to have good collisions. What defines a good collision?

Important Laws – 40 • The correct orientation and the enough energy/speed

Important Laws - 50 • Draw a picture of particles reacting, according to collision theory

Important Laws – 50

Endothermic or Exothermic? - 10 • The reaction is cold to touch

Endothermic or Exothermic? – 10 • Endothermic

Endothermic or Exothermic? - 20 • The reaction releases energy

Endothermic or Exothermic? – 20 • Exothermic

Endothermic or Exothermic? - 30 • The potential energy diagram looks like this:

Endothermic or Exothermic? – 30 • Exothermic

Endothermic or Exothermic? - 40 • The starting energy for the reactants was 90 k. J and the ending energy for the reactants was 120 k. J

Endothermic or Exothermic? – 40 • Endothermic
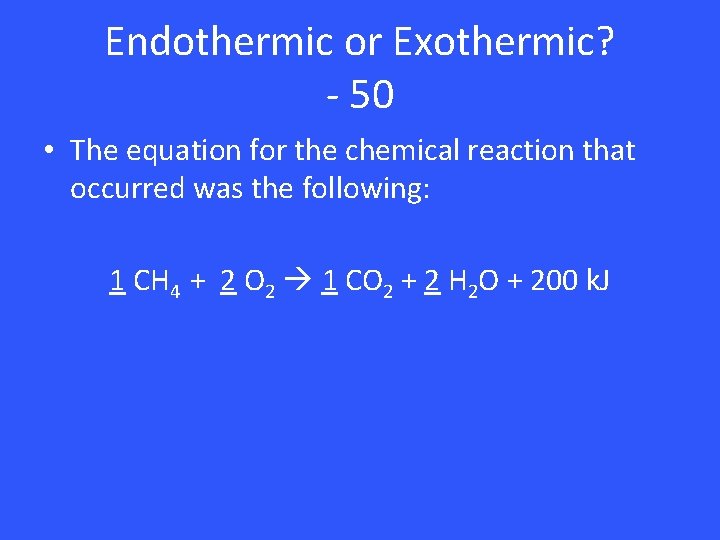
Endothermic or Exothermic? - 50 • The equation for the chemical reaction that occurred was the following: 1 CH 4 + 2 O 2 1 CO 2 + 2 H 2 O + 200 k. J

Endothermic or Exothermic? – 50 • Exothermic (Needs energy on products to balance, thus reaction released energy)

Potential Energy Diagrams - 10 • What do you call the two circled areas?

Potential Energy Diagrams – 10 • Left Circle: Reactants • Right Circle: Products
Potential Energy Diagrams - 20 • Draw a line on the graph representing the change in enthalpy

Potential Energy Diagrams – 20 • Middle Red Line

Potential Energy Diagrams - 30 • Determine the energy of the products from the potential energy diagram:

Potential Energy Diagrams – 30

Potential Energy Diagrams - 40 • What is the name of the circled area in the graph?

Potential Energy Diagrams – 40 • Activation Energy: Energy needed to start the reaction

Potential Energy Diagrams - 50 • Draw the potential energy diagram for this: • You performed a chemical reaction. Your reactants started with 500 k. J. After, your products had 300 k. J. It took 100 k. J to activate the reaction.

Potential Energy Diagrams – 50
- Slides: 51