REACh The New Toxicology Frontier REACh Registration Evaluation



























- Slides: 38

REACh: The New Toxicology Frontier REACh Registration, Evaluation and Authorization of Chemicals n! ctio i r t Res and Ohio Valley SOT Wednesday, August 26, 2009 1

Presenters Jennifer Galvin, Ph. D, DABT, CIH Manager, Industrial Hygiene & Toxicology Conoco. Phillips Tracy Hammon, MS, DABT Director, Product Safety Conoco. Phillips 2

Overview • • • What is REACh? Why REACh? Goals of REACh Impacts of REACh General Information Requirements Technical Dossier Chemical Safety Report Extended SDS 3

What is REACh? • New EU Chemicals Regulation • REACh : Registration, Evaluation, Authorization and Restriction of Chemicals • REACh replaces 40 existing EU Chemical Regulations and Directives 4

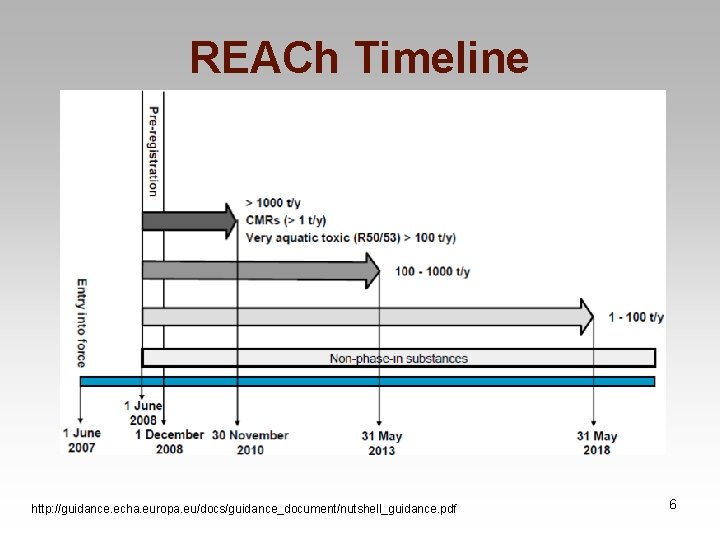
Stages of REACh • Pre-registration: by December 2008 • Registration: for substances ≥ 1 ton/yr • Evaluation: for high volume substances which are of highest concern • Authorization: only for substances of highest concern • Restriction: the Safety net The European Chemicals Agency (ECHA) was established to manage the system 5

REACh Timeline http: //guidance. echa. europa. eu/docs/guidance_document/nutshell_guidance. pdf 6

Why REACh? • Lack of knowledge about chemical hazards on the EU market. • Prior legislation was regarded as slow & burdensome. • Incomplete information on existing chemicals vs. new chemicals. • The burden of proof was on regulators. 7

Goals of REACh • Enhance transparency and efficiency • Close data gaps between existing & new substances. • Manage and control potential hazards and risks to human health and the environment from the manufacture, import and use of chemicals within the EU 8

Impacts of REACh • REACh is a global business issue that will drive major changes in the way chemical businesses are organized • REACh has the potential to be a major threat to supply chain continuity • Clear and decisive leadership and management is needed 9

Businesses need to understand how valuable these substances are to them, and plan to make effective business decisions based on this knowledge. Decisions made today will impact future business practices 10

No data = No market 11

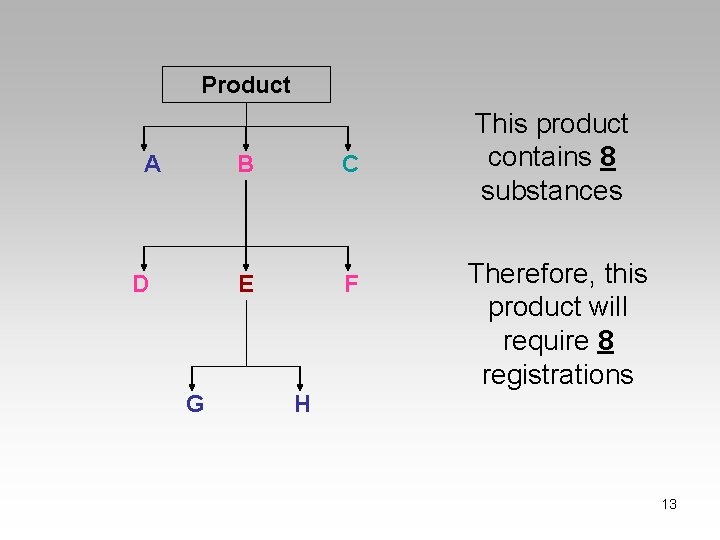
REACh is a substance-specific regulation PRODUCT 12

Product A D G B C E F This product contains 8 substances Therefore, this product will require 8 registrations H 13

Multiple Expert Requirements • • • • lawyers lobbyists communicators IT-specialists regulatory experts physicians Toxicologists hygienists researchers process engineers purchasers & logistics marketing network Export/Import coordinators 14

Impacts of REACh • Burden of proof has shifted to industry • Industry will have to prepare a comprehensive document including: – Hazards – Risk management 15

Classification & Labeling • Under the new Global Harmonized System – Each substance will be required to undergo classification • Under REACh, this classification will be documented in the registration dossier 16

Identified Uses • Use of the substance must be included in the registration • If substance is hazardous, exposures need to be assessed 17

REACh Phases Pre-Registration SIEF Formation Registration Evaluation Authorization Restriction 18

Pre-Registration • Phase-in Substances • Pre-registration deadline December 2008 • Approx. 143, 000 existing substances were pre-registered – ECHA received about fifteen times more preregistrations than expected 19

SIEF • Substance Information Exchange Forum • Purpose: – Data sharing (compulsory) – Agreement on Classification and Labeling 20

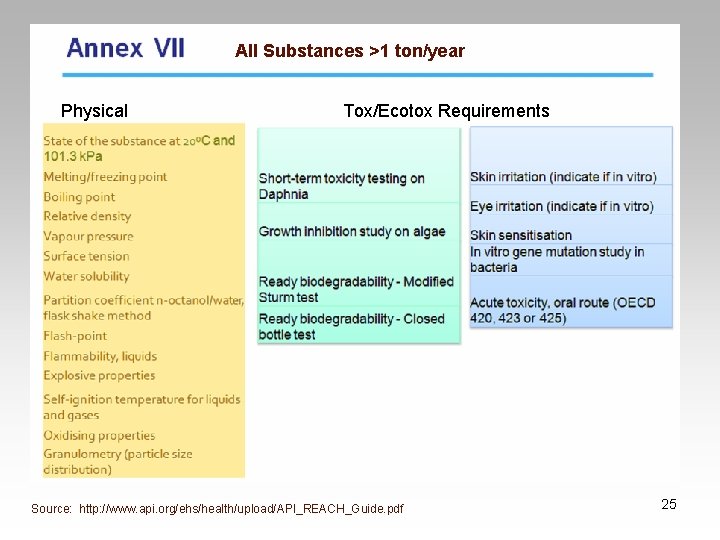
Registration • Substances > 1 ton/year • Develop Technical Dossier – Reduced requirements for intermediates • Chemical Safety Assessment • Classification 21

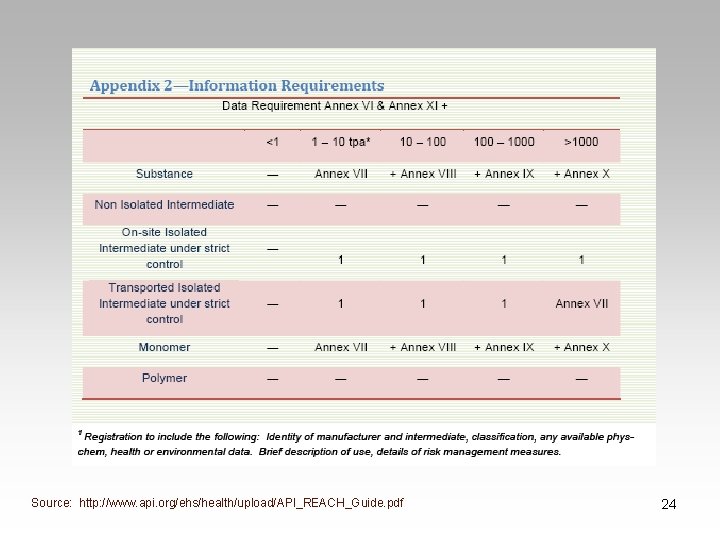
Technical Dossier • Required for registration • Information requirements – dependent on tonnage band • Contents: – Information on manufacture & use of substance – Physical characteristics, toxicological & ecotoxicological properties – Proposals for testing if appropriate – Indication of information submitted that should not be made available on internet & why 22

Proposals for testing…. if appropriate This is not a regulation that requires testing 1. 2. 3. 4. Provide the data available Do the risk assessment Determine data gaps Drive testing requirements 23

Source: http: //www. api. org/ehs/health/upload/API_REACH_Guide. pdf 24

All Substances >1 ton/year Physical Tox/Ecotox Requirements Source: http: //www. api. org/ehs/health/upload/API_REACH_Guide. pdf 25

+ 10 - 100 tons/year Source: http: //www. api. org/ehs/health/upload/API_REACH_Guide. pdf 26

+ 100 - 1000 tons/year Source: http: //www. api. org/ehs/health/upload/API_REACH_Guide. pdf 27

+ > 1000 tons/year Source: http: //www. api. org/ehs/health/upload/API_REACH_Guide. pdf 28

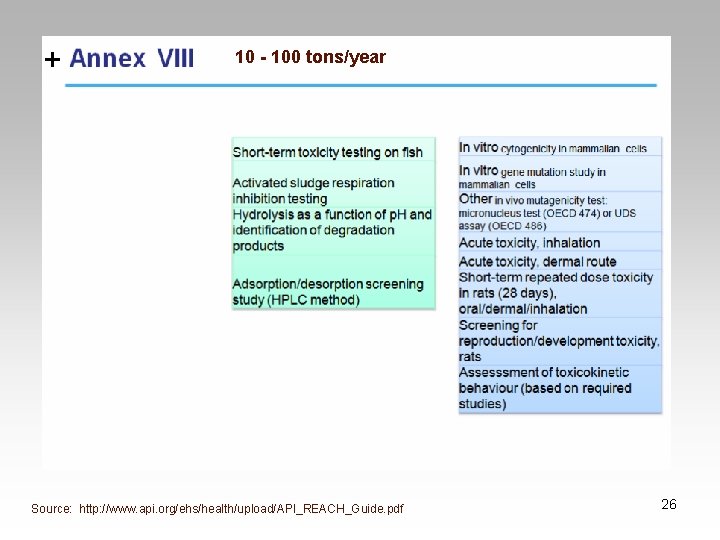
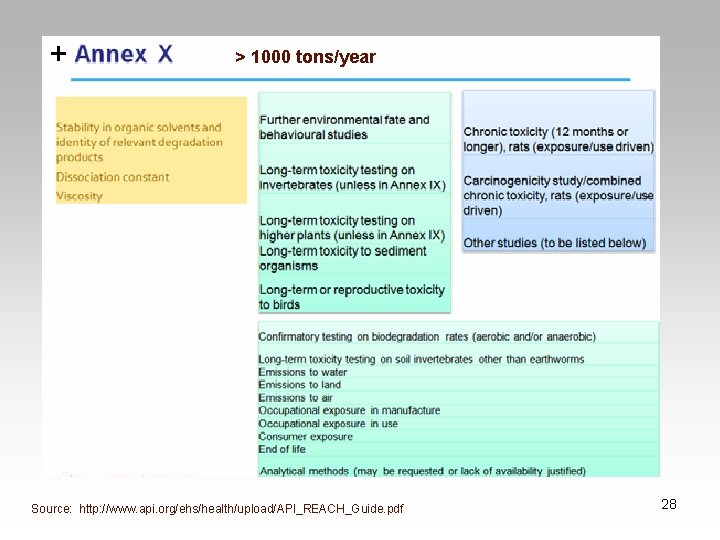
Evaluation • Substances > 10 tons/year • Chemical Safety Assessment • Requires detailed Chemical Safety Report – Required if a substance is dangerous, PBT or a v. Pv. B – Risk characterization on exposures from intended uses – Risk Characterization 29

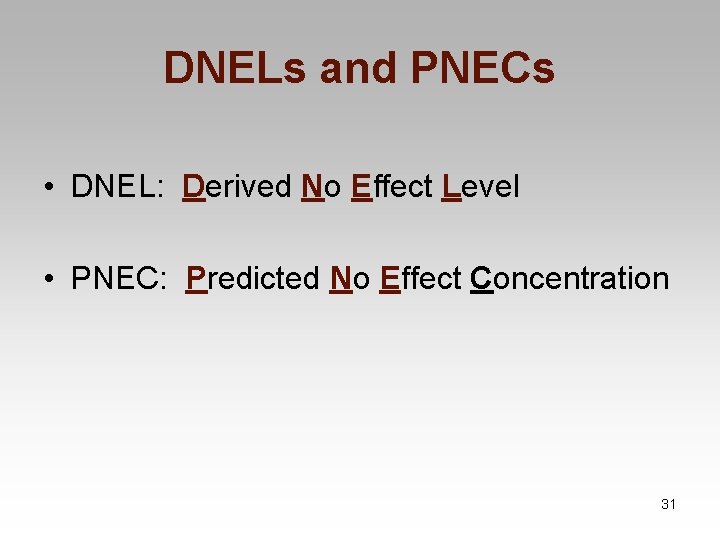
Chemical Safety Assessment • Safety (physico-chemical) • Human health • Evaluate data • Classification and labelling • Establish Derived No-Effect Level (DNEL) • Environmental • Evaluate data • Classification and labelling • Establish Predicted No-Effect Concentration (PNEC) 30

DNELs and PNECs • DNEL: Derived No Effect Level • PNEC: Predicted No Effect Concentration 31

Exposure Scenarios • Manufacture and intended uses – • • • substance life cycle (including disposal/recycling) Include • processes and tasks • frequency and duration (how often and how long? ) • operational conditions • representative exposure data or modelling data • What risk management measures are required? For each human population exposed – Workers – Consumers – Indirect Included as an appendix to the Safety Data Sheet (SDS) – Now called an Extended Safety Data Sheet (e. SDS) 32

Risk Management Measures • Must address workers, consumers, and the general public • For workers, consider… • Hierarchy of control • Principles of Good Control Practice (COSHH) • All routes of exposure (e. g. , inhalation, dermal, ingestion) • Determine residual risk • Risk Characterization – compare exposure with the relevant DNEL 33

Environmental Assessment PBT Persistent, Bioaccumulative & Toxic v. Pv. B very Persistent & very Bioaccumulative 34

Extended Safety Data Sheet (e. SDS) • Used to communicate hazard down the supply chain • Increased requirements compared to current EU standard • Exposure scenarios 35

Authorization • Required for all substances of very high concern • Approx. 1, 500 -2, 000 substances • Time limited • Substances of very high concern are: • • CMR (Carcinogen, Mutagen or Toxic for Reproduction Cat. 1 or 2) PBT (Persistent & Bioaccumulative & Toxic) or v. Pv. B (Very Persistent & Very Bioaccumulative & Toxic) substances of equivalent level of concern • Applicants must • demonstrate adequate control of risks or that socio-economic benefits outweigh risks • develop substitution plans or inform on research to find alternatives • Restrictions can be applied to any substance 36

Conclusion • Global Impact of REACh – US – TSCA Reform – China – ROHS – Taiwan – Canada The Fall-Out 37

38
 Frontier detectors for frontier physics
Frontier detectors for frontier physics Frontiern
Frontiern Jfk cartoon
Jfk cartoon Chapter 20 section 2 the new frontier
Chapter 20 section 2 the new frontier New frontier camelot
New frontier camelot Chapter 28 section 2 the new frontier
Chapter 28 section 2 the new frontier New frontier jfk
New frontier jfk New frontier camelot
New frontier camelot Chapter 15 the new frontier and the great society
Chapter 15 the new frontier and the great society New frontier vs great society
New frontier vs great society The new frontier and great society
The new frontier and great society New frontier vs great society
New frontier vs great society Kennedys new frontier
Kennedys new frontier Digitalgujarat.gov.in new registration
Digitalgujarat.gov.in new registration User registration process flow diagram
User registration process flow diagram Calaters global
Calaters global Ogras bihar
Ogras bihar Toxicology is the study of
Toxicology is the study of Toxicology excellence for risk assessment
Toxicology excellence for risk assessment Important people in forensics
Important people in forensics Reinsch test
Reinsch test Forensics toxicology definition
Forensics toxicology definition Definition of forensic toxicology
Definition of forensic toxicology Define environmental toxicology
Define environmental toxicology Chapter 8 toxicology poisons and alcohol
Chapter 8 toxicology poisons and alcohol Chapter 8 toxicology test
Chapter 8 toxicology test Toxicology management
Toxicology management Washington state patrol toxicology lab
Washington state patrol toxicology lab Forensic toxicology lab activity
Forensic toxicology lab activity Toxicology definition
Toxicology definition Toxicology and applied pharmacology
Toxicology and applied pharmacology North carolina medical examiner
North carolina medical examiner Toxicology
Toxicology Examples of toxicology
Examples of toxicology Therapeutic index
Therapeutic index Toxicology
Toxicology Toxicology defination
Toxicology defination Which is more toxic
Which is more toxic Annual review of pharmacology and toxicology
Annual review of pharmacology and toxicology