REACH STANDARD INFORMATION REQUIRED According to Annex VII


















- Slides: 22

REACH STANDARD INFORMATION REQUIRED According to Annex VII and VIII 1 -100 tonnes

ANNEX VII STANDARD INFORMATION REQUIREMENTS FOR SUBSTANCES MANUFACTURED OR IMPORTED IN QUANTITIES OF 1 TONNE OR MORE ANNEX VIII STANDARD INFORMATION REQUIREMENTS FOR SUBSTANCES MANUFACTURED OR IMPORTED IN QUANTITIES OF 10 TONNES OR MORE (up 100 tonnes)

Part A: Methods for the determination of the physico-chemical properties A. 1 MELTING/FREEZING TEMPERATURE A. 2 BOILING TEMPERATURE A. 3 RELATIVE DENSITY A. 4 VAPOUR PRESSURE A. 5 SURFACE TENSION A. 6 WATER SOLUBILITY A. 8 PARTITION COEFFICIENT A. 9 FLASH-POINT A. 10 FLAMMABILITY (SOLIDS) A. 11 FLAMMABILITY (GASES) A. 12 FLAMMABILITY (CONTACT WITH WATER) A. 13 PYROPHORIC PROPERTIES OF SOLIDS AND LIQUIDS A. 14 EXPLOSIVE PROPERTIES A. 15 AUTO-IGNITION TEMPERATURE (LIQUIDS AND GASES) A. 16 RELATIVE SELF-IGNITION TEMPERATURE FOR SOLIDS A. 17 OXIDIZING PROPERTIES (SOLIDS) A. 18 NUMBER - AVERAGE MOLECULAR WEIGHT AND MOLECULAR WEIGHT DISTRIBUTION OF POLYMERS A. 19 LOW MOLECULAR WEIGHT CONTENT OF POLYMERS A. 20 SOLUTION / EXTRACTION BEHAVIOUR OF POLYMERS IN WATER A. 21 OXIDISING PROPERTIES (LIQUIDS)

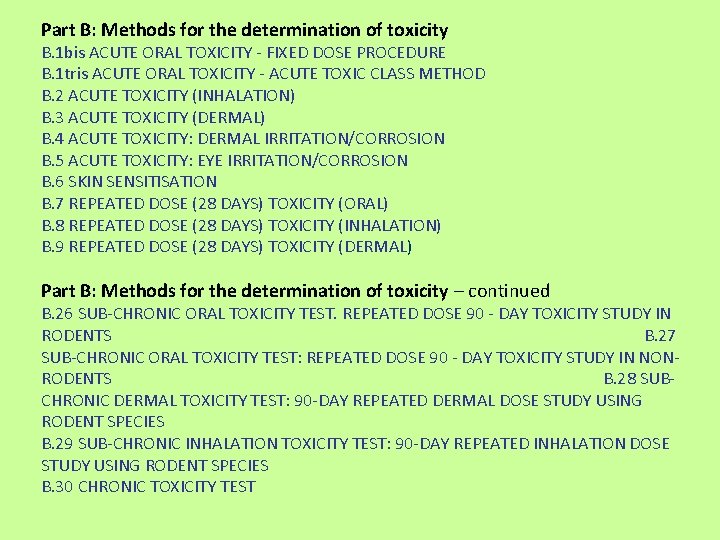
Part B: Methods for the determination of toxicity B. 1 bis ACUTE ORAL TOXICITY - FIXED DOSE PROCEDURE B. 1 tris ACUTE ORAL TOXICITY - ACUTE TOXIC CLASS METHOD B. 2 ACUTE TOXICITY (INHALATION) B. 3 ACUTE TOXICITY (DERMAL) B. 4 ACUTE TOXICITY: DERMAL IRRITATION/CORROSION B. 5 ACUTE TOXICITY: EYE IRRITATION/CORROSION B. 6 SKIN SENSITISATION B. 7 REPEATED DOSE (28 DAYS) TOXICITY (ORAL) B. 8 REPEATED DOSE (28 DAYS) TOXICITY (INHALATION) B. 9 REPEATED DOSE (28 DAYS) TOXICITY (DERMAL) Part B: Methods for the determination of toxicity – continued B. 26 SUB-CHRONIC ORAL TOXICITY TEST. REPEATED DOSE 90 - DAY TOXICITY STUDY IN RODENTS B. 27 SUB-CHRONIC ORAL TOXICITY TEST: REPEATED DOSE 90 - DAY TOXICITY STUDY IN NONRODENTS B. 28 SUBCHRONIC DERMAL TOXICITY TEST: 90 -DAY REPEATED DERMAL DOSE STUDY USING RODENT SPECIES B. 29 SUB-CHRONIC INHALATION TOXICITY TEST: 90 -DAY REPEATED INHALATION DOSE STUDY USING RODENT SPECIES B. 30 CHRONIC TOXICITY TEST

Part B: Methods for the determination of toxicity – continued B. 31 TERATOGENICITY TEST – RODENT AND NON-RODENT B. 32 CARCINOGENICITY TEST B. 33 COMBINED CHRONIC TOXICITY/CARCINOGENICITY TEST B. 34 ONE-GENERATION REPRODUCTION TOXICITY TEST B. 35 TWO GENERATION REPRODUCTION TOXICITY TEST B. 36 TOXICOKINETICS B. 37 DELAYED NEUROTOXICITY OF ORGANOPHOSPHORUS SUBSTANCES FOLLOWING ACUTE EXPOSURE B. 38 DELAYED NEUROTOXICITY OF ORGANOPHOSPHORUS SUBSTANCES 28 DAY REPEATED DOSE STUDY B. 40 SKIN CORROSION (IN VITRO) B. 41 PHOTOTOXICITY - IN VITRO 3 T 3 NRU PHOTOTOXICITY TEST B. 42 SKIN SENSITISATION: LOCAL LYMPH NODE ASSAY B. 43 NEUROTOXICITY STUDY IN RODENTS

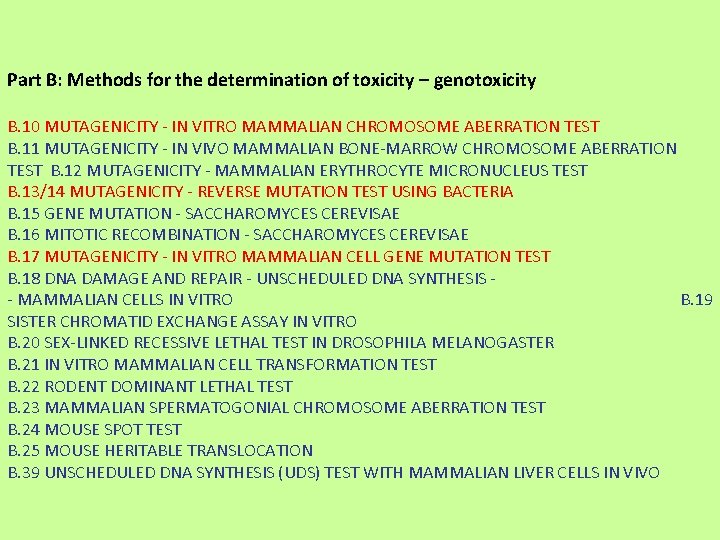
Part B: Methods for the determination of toxicity – genotoxicity B. 10 MUTAGENICITY - IN VITRO MAMMALIAN CHROMOSOME ABERRATION TEST B. 11 MUTAGENICITY - IN VIVO MAMMALIAN BONE-MARROW CHROMOSOME ABERRATION TEST B. 12 MUTAGENICITY - MAMMALIAN ERYTHROCYTE MICRONUCLEUS TEST B. 13/14 MUTAGENICITY - REVERSE MUTATION TEST USING BACTERIA B. 15 GENE MUTATION - SACCHAROMYCES CEREVISAE B. 16 MITOTIC RECOMBINATION - SACCHAROMYCES CEREVISAE B. 17 MUTAGENICITY - IN VITRO MAMMALIAN CELL GENE MUTATION TEST B. 18 DNA DAMAGE AND REPAIR - UNSCHEDULED DNA SYNTHESIS - MAMMALIAN CELLS IN VITRO B. 19 SISTER CHROMATID EXCHANGE ASSAY IN VITRO B. 20 SEX-LINKED RECESSIVE LETHAL TEST IN DROSOPHILA MELANOGASTER B. 21 IN VITRO MAMMALIAN CELL TRANSFORMATION TEST B. 22 RODENT DOMINANT LETHAL TEST B. 23 MAMMALIAN SPERMATOGONIAL CHROMOSOME ABERRATION TEST B. 24 MOUSE SPOT TEST B. 25 MOUSE HERITABLE TRANSLOCATION B. 39 UNSCHEDULED DNA SYNTHESIS (UDS) TEST WITH MAMMALIAN LIVER CELLS IN VIVO

Part C: Methods for the determination of ecotoxicity C. 1 ACUTE TOXICITY FOR FISH C. 2 ACUTE TOXICITY FOR DAPHNIA C. 3 ALGAL INHIBITION TEST C. 4 BIODEGRADATION: DETERMINATION OF THE "READY" BIODEGRADABILITY C. 4 -A DISSOLVED ORGANIC CARBON (DOC) DIE-AWAY TEST C. 4 -B MODIFIED OECD SCREENING TEST C. 4 -C CARBON DIOXIDE EVOLUTION TEST C. 4 -D MANOMETRIC RESPIROMETRY TEST C. 4 -E CLOSED BOTTLE TEST C. 4 -F MITI TEST C. 5 DEGRADATION : BIOCHEMICAL OXYGEN DEMAND C. 6 DEGRADATION: CHEMICAL OXYGEN DEMAND C. 7 DEGRADATION: ABIOTIC DEGRADATION: HYDROLYSIS AS A FUNCTION OF PH C. 8 TOXICITY FOR EARTHWORMS : ARTIFICIAL SOIL TEST C. 9 BIODEGRADATION: ZAHN - WELLENS TEST C. 10 BIODEGRADATION: ACTIVATED SLUDGE SIMULATION TEST C. 11 BIODEGRADATION: ACTIVATED SLUDGE RESPIRATION INHIBITION TEST C. 12 BIODEGRADATION: MODIFIED SCAS TEST

Part C: Methods for the determination of ecotoxicity – continued C. 13 BIOCONCENTRATION: FLOW-THROUGH FISH TEST C. 14 FISH JUVENILE GROWTH TEST C. 15 FISH, SHORT-TERM TOXICITY TEST ON EMBRYO AND SAC-FRY STAGES C. 16 HONEYBEES - ACUTE ORAL TOXICITY TEST C. 17 HONEYBEES - ACUTE CONTACT TOXICITY TEST C. 18 ADSORPTION/DESORPTION USING A BATCH EQUILIBRIUM METHOD C. 19 ESTIMATION OF THE ADSORPTION COEFFICIENT (KOC) ON SOIL AND ON SEWAGE SLUDGE USING HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) C. 20 DAPHNIA MAGNA REPRODUCTION TEST C. 21 SOIL MICROORGANISMS: NITROGEN TRANSFORMATION TEST C. 22 SOIL MICROORGANISMS: CARBON TRANSFORMATION TEST C. 23 AEROBIC AND ANAEROBIC TRANSFORMATION IN SOIL C. 24 AEROBIC AND ANAEROBIC TRANSFORMATION IN AQUATIC SEDIMENT SYSTEMS

8. 1. Skin irritation or skin corrosion The assessment of this endpoint shall comprise the following consecutive steps: (1) an assessment of the animal data, (2) an assessment of the (3) in vitro study for skin (4) in vitro study for skin available human and acid or alkaline reserve, corrosion, irritation.

8. 1. Skin irritation or skin corrosion 8. 1. 1. In vivo skin irritation

8. 2. Eye irritation The assessment of this endpoint shall comprise the following consecutive steps: (1) an assessment of the available human and animal data, (2) an assessment of the acid or alkaline reserve, (3) in vitro study for eye irritation.

8. 2. Eye irritation 8. 2. 1. In vivo eye irritation

8. 3. Skin sensitisation The assessment of this endpoint shall comprise the following consecutive steps: (1) an assessment of the available human, animal and alternative data, (2) In vivo testing. The murine Local Lymph Node Assay (LLNA) is the first- choice method for in vivo testing

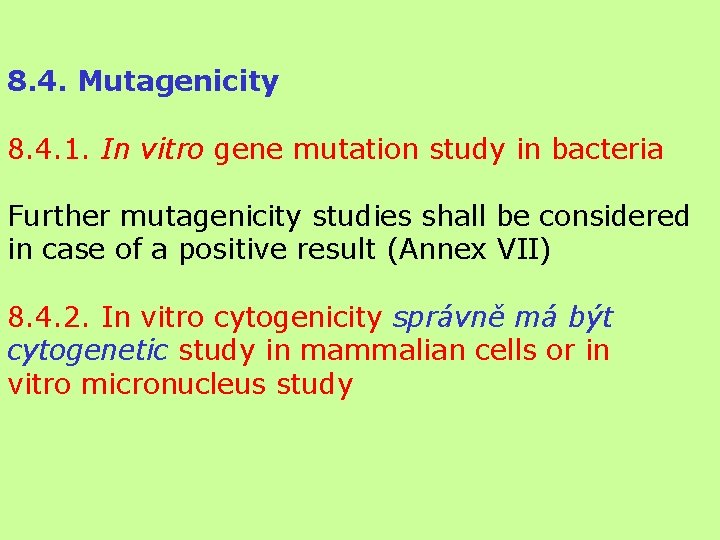
8. 4. Mutagenicity 8. 4. 1. In vitro gene mutation study in bacteria Further mutagenicity studies shall be considered in case of a positive result (Annex VII) 8. 4. 2. In vitro cytogenicity správně má být cytogenetic study in mammalian cells or in vitro micronucleus study

8. 4. Mutagenicity 8. 4. 3. In vitro gene mutation study in mammalian cells If negative results of studies under 8. 4. 1 and 8. 4. 2

8. 5 Acute toxicity 8. 5. The study/ ies do( es) not generally need to be conducted if: – -the substance is classified as corrosive to the skin. 8. 5. 1. By oral route 8. 5. 2 By inhalation 8. 5. 1. By dermal route

8. 6. Repeated dose toxicity 8. 6. 1. Short- term repeated dose toxicity study (28 days) one species, male and female, most appropriate route of administration, having regard to the likely route of human exposure.

8. 7. Reproductive toxicity 8. 7. 1. Screening for reproductive/ developmental toxicity, one species (OECD 421 or 422), -if there is no evidence from available information on structurally related substances, from (Q)SAR estimates, or from in vitro methods tha the substance may be a developmental toxicant

8. 8. Toxicokinetics 8. 8. 1. Assessment of the toxicokinetic behaviour of the substance to the extent that can be derived from the relevant available information

9. 1. Aquatic toxicity 9. 1. 1. Short- term toxicity testing on invertebrates (preferred species Daphnia) 9. 1. 2. Growth inhibition study aquatic plants (algae preferred) 9. 1. 3. Short- term toxicity testing on fish 9. 1. 4. Activated sludge respiration inhibition testing

9. 2. Degradation 9. 2. 1. Biotic 9. 2. 1. 1. Ready biodegradability 9. 2. 2. Abiotic 9. 2. 2. 1. Hydrolysis as a function of p. H

9. 3. Fate and behaviour in the environment 9. 3. 1. Adsorption/ desorption screening
 Reach annex vii testing
Reach annex vii testing What are the six principal views
What are the six principal views Tetraphosphorous decasulfide formula
Tetraphosphorous decasulfide formula Fernando vii
Fernando vii De ce nu-mi vii
De ce nu-mi vii Pedagogika tarixi fan sifatida ppt
Pedagogika tarixi fan sifatida ppt ჰალოგენები
ჰალოგენები Pasari carenate
Pasari carenate Facial nerve sensory test
Facial nerve sensory test Cn 12 test
Cn 12 test Henryk vii tudor
Henryk vii tudor Animalele se pot inmulti prin
Animalele se pot inmulti prin Monarchs of england
Monarchs of england King henry viii family tree
King henry viii family tree 100 people surveyed
100 people surveyed Lettera vii platone
Lettera vii platone Nerf 7 bis
Nerf 7 bis Song vii answer key
Song vii answer key 6/1996. (vii. 16.) müm rendelet
6/1996. (vii. 16.) müm rendelet Article v the teachers and the profession
Article v the teachers and the profession 10/2015 (vii. 30) hm
10/2015 (vii. 30) hm Fernando vii absolutismo
Fernando vii absolutismo Ecuacion indicial
Ecuacion indicial