REACH and Downstream Users Marie Mc Carthy REACH

















- Slides: 27

REACH and Downstream Users Marie Mc. Carthy REACH GI Inspector Health and Safety Authority H S &A HEALTH AND SAFETY AUTHORITY

Am I a Downstream User ? H S &A HEALTH AND SAFETY AUTHORITY

Registration As it applies to Downstream Users (DUs) H S &A HEALTH AND SAFETY AUTHORITY

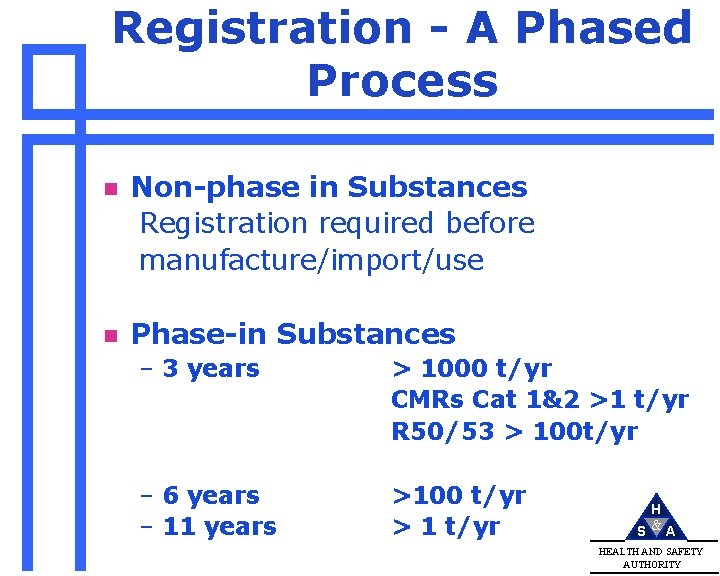
Registration - A Phased Process n Non-phase in Substances Registration required before manufacture/import/use n Phase-in Substances – 3 years > 1000 t/yr CMRs Cat 1&2 >1 t/yr R 50/53 > 100 t/yr – 6 years – 11 years >100 t/yr > 1 t/yr H S &A HEALTH AND SAFETY AUTHORITY

Registration of Substances in Articles Required where § Substance is present at > 1 t/yr per producer or importer § Substance is intended to be released under normal or forseeable conditions Unless substance has already been registered for that use H S &A HEALTH AND SAFETY AUTHORITY

Notification of Substances in Articles Required where § Substance meets criteria in Art 54 and is identified according to Art 56(1) § Substance present at > 1 t/yr/M or I § Substance present at > 0. 1% w/v Unless M/I can exclude exposure to humans & environment under normal & forseeable conditions of use. H S &A HEALTH AND SAFETY AUTHORITY

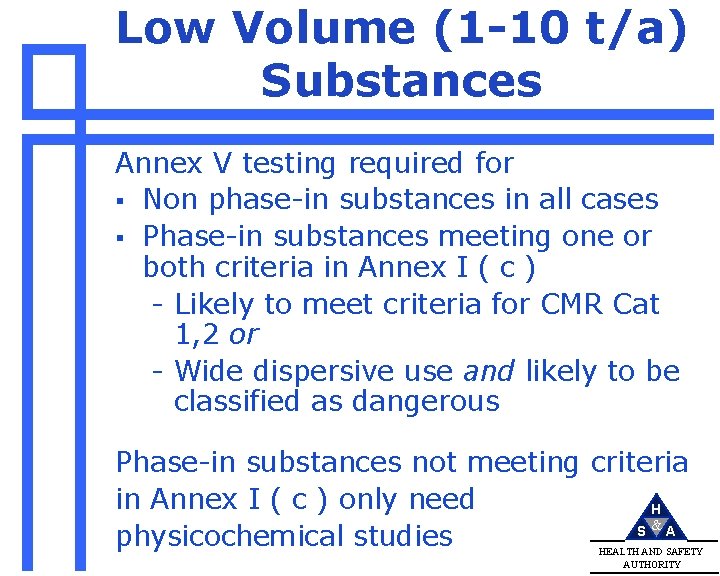
Low Volume (1 -10 t/a) Substances Annex V testing required for § Non phase-in substances in all cases § Phase-in substances meeting one or both criteria in Annex I ( c ) - Likely to meet criteria for CMR Cat 1, 2 or - Wide dispersive use and likely to be classified as dangerous Phase-in substances not meeting criteria in Annex I ( c ) only need H S &A physicochemical studies HEALTH AND SAFETY AUTHORITY

Tools used by Industry § § CSA used to determine which risk management measures and operational conditions are necessary to ensure risks to human health and environment are adequately controlled CSR used to document the appropriate risk management measures and operating conditions to ensure adequate control Exposure scenario (ES) is a description of the set of conditions for use of a substance so that risks are adequately controlled. This must reflect the outcome of the CSA, be documented in the CSR and annexed to SDS H SDS used to communicate risk S &A management measures downstream. HEALTH AND SAFETY AUTHORITY

Chemical Safety Assessment n n n CSA (Annex I) at > 10 t/yr Registrant must ensure that risks are adequately controlled for manufacture and/or each identified use Must specify risk management measures for each exposure scenario DU has a right to identify use to M/I includes this in his CSR if he can support the use DU may choose not to identify use H but may have to notify Chemicals S &A Agency & perform CSA HEALTH AND SAFETY AUTHORITY

Exposure Scenario - conditions ensuring adequate control § § § § Physicochemical characteristics Process description Operating conditions Risk management measures Populations exposed Must cover entire life cycle including use in articles, consumer use, waste disposal etc. Process-specific conditions >>>> broad, generic ESs covering H multiple uses/substances S &A HEALTH AND SAFETY AUTHORITY

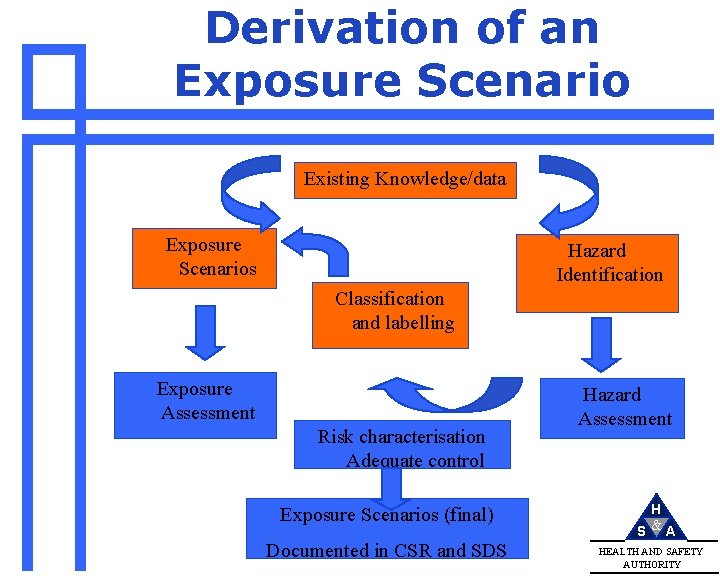
Derivation of an Exposure Scenario Existing Knowledge/data Exposure Scenarios Hazard Identification Classification and labelling Exposure Assessment Risk characterisation Adequate control Exposure Scenarios (final) Documented in CSR and SDS Hazard Assessment H S &A HEALTH AND SAFETY AUTHORITY

Substance X in Paints for Brushing/Application by Roller Standardised exposure scenario for group of substances outlining • • Protective clothing Maximum periods of exposure Cleaning of brushes/rollers etc Disposal of waste H S &A HEALTH AND SAFETY AUTHORITY

Substance X used in Spraypainting of Cars Process- specific exposure scenario outlining • Maximum periods of exposure • Minimum requirements for the equipment (booth, breathing apparatus) • Filtering efficiency • Cleaning frequency and procedures • Protective clothing • Hygiene measures H • Waste disposal & S A HEALTH AND SAFETY AUTHORITY

Is my use covered by my supplier’s registration ? H S &A HEALTH AND SAFETY AUTHORITY

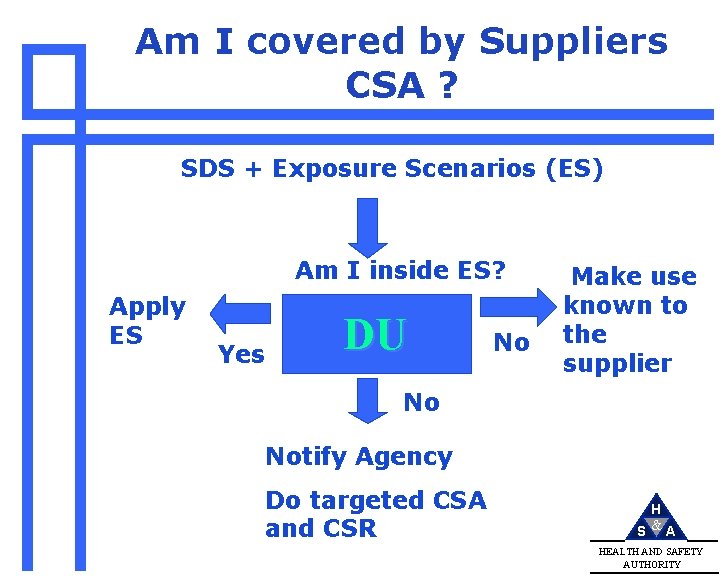
Am I covered by Suppliers CSA ? SDS + Exposure Scenarios (ES) Am I inside ES? Apply ES Yes DU No Make use known to the supplier No Notify Agency Do targeted CSA and CSR H S &A HEALTH AND SAFETY AUTHORITY

Do I need to act? DU takes no further action in the following situations: § He is using less than 1 t/yr § He is operating within the conditions of an exposure scenario communicated via SDS and has implemented recommended RMM on his site § SDS not required for substance (e. g. not dangerous) § CSR not required for M/I ( e. g. < 10 H tonnes/yr) S &A § He is using it for PPORD HEALTH AND SAFETY AUTHORITY

I need to act The DU must complete a CSR and report to Agency in the following situations: § CSR required and § He is operating outside exposure scenario communicated in an SDS or § He has chosen to keep his use secret or M/I has not taken account of it § Methodology for DU CSA in Annex XI § Must implement appropriate Risk Management measures (RMM) on own H site and inform DUs of S &A appropriate RRM for their use HEALTH AND SAFETY AUTHORITY

DU Report § DU Report is not a registration § Limited information on identity of DU and supplier, identity of substance and generic use description § In limited circumstances, testing proposal may be required – dossier evaluation § Reporting not required for small quantities (< 1 tonne) H S &A HEALTH AND SAFETY AUTHORITY

Information Flow Through the Supply Chain H S &A HEALTH AND SAFETY AUTHORITY

Information flow through the Supply Chain § SDS main tool Extended scope & role § All dangerous substances and prepartions placed on the market § PBTs/v. Pv. Bs identified by Annex XII criteria § Must be consistent with CSA § Exposure scenarios annexed to SDS § Information flow up and down supply chain H S &A HEALTH AND SAFETY AUTHORITY

Roles for DUs under REACH § Check compliance with suppliers exposure scenario § If not covered, and where necessary, report to Agency and carry out a CSA § Comply with any authorisation and restriction conditions § Communication upstream & downstream H S &A HEALTH AND SAFETY AUTHORITY

Supports for DUs & SMEs § § § Guidance for downstream users (RIP 3. 5) Guidance on articles (RIP 3. 8) Tools for SMEs Trade Associations - development of standardised use descriptions National helpdesk – Health and Safety Authority Agency helpdesk H S &A HEALTH AND SAFETY AUTHORITY

Benefits for DUs & SMEs § Improved access to information § Better quality of information § Informed decision-making § Improved worker safety § Reduced liability and compensation costs § Increased innovation H S &A HEALTH AND SAFETY AUTHORITY

Risks for SMEs and DUs § Increased costs § Withdrawl of substances § Disclosure of confidential business information H S &A HEALTH AND SAFETY AUTHORITY

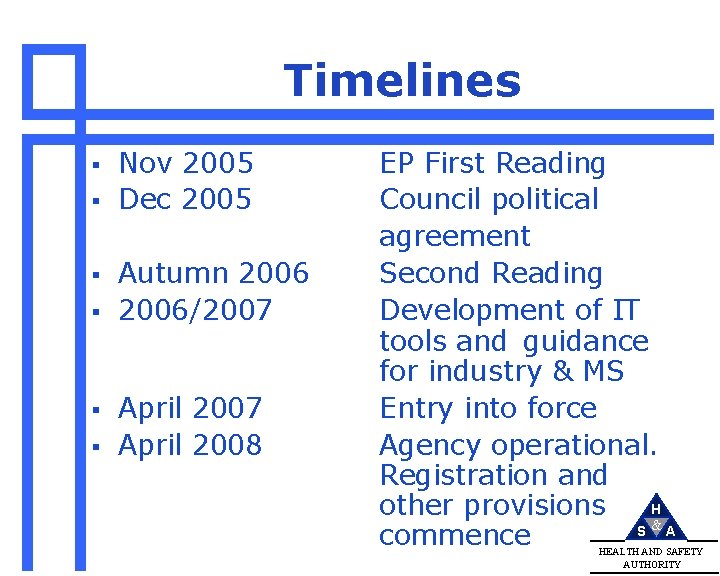
Timelines § § § Nov 2005 Dec 2005 Autumn 2006/2007 April 2008 EP First Reading Council political agreement Second Reading Development of IT tools and guidance for industry & MS Entry into force Agency operational. Registration and H other provisions S &A commence HEALTH AND SAFETY AUTHORITY

What should I do now ? § Monitor development of REACH and Guidance documents § Assess your use of chemicals § Think about implications of REACH for your business § Dialogue a. s. a. p!!! H S &A HEALTH AND SAFETY AUTHORITY

Useful Websites Commission Websites http: //europa. eu. int/comm/enterprise/rea ch http: //europa. eu. int/comm/environment/ chemicals/reach ECB Website http: //ecb. jrc. it/ Health and Safety Authority Website www. hsa. ie Queries on REACH wcu@hsa. ie H S &A HEALTH AND SAFETY AUTHORITY