RBC DISORDERS Anemia Reduction of total RBC MASS


RBC DISORDERS

Anemia • Reduction of total RBC MASS below average levels • Reduction of oxygen carrying capacity of the blood, leads to tissue hypoxia • Practically, measured by Hemoglobin concentration, and Hematocrit (ratio of packed RBCs to total blood volume)
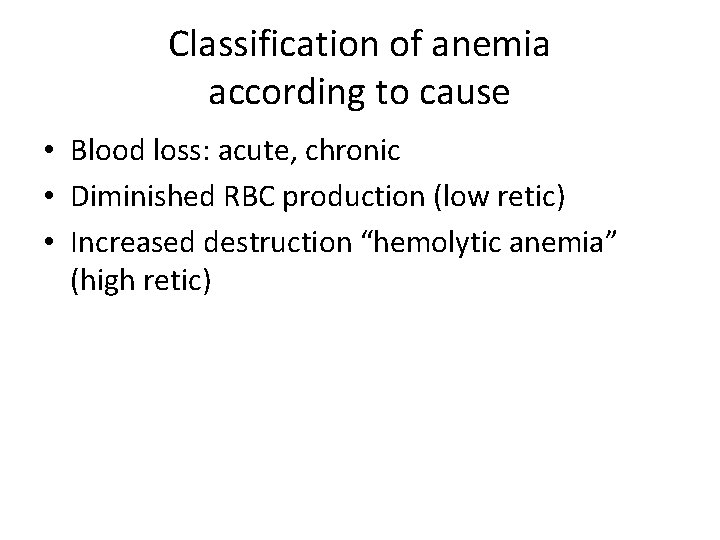
Classification of anemia according to cause • Blood loss: acute, chronic • Diminished RBC production (low retic) • Increased destruction “hemolytic anemia” (high retic)
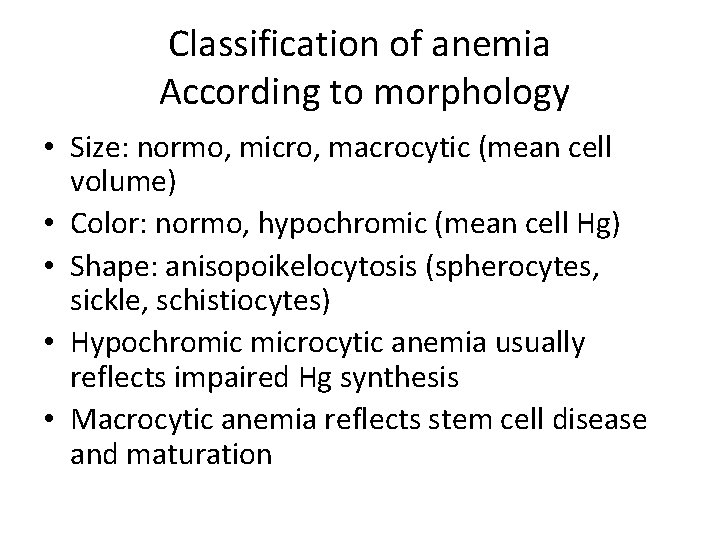
Classification of anemia According to morphology • Size: normo, micro, macrocytic (mean cell volume) • Color: normo, hypochromic (mean cell Hg) • Shape: anisopoikelocytosis (spherocytes, sickle, schistiocytes) • Hypochromic microcytic anemia usually reflects impaired Hg synthesis • Macrocytic anemia reflects stem cell disease and maturation
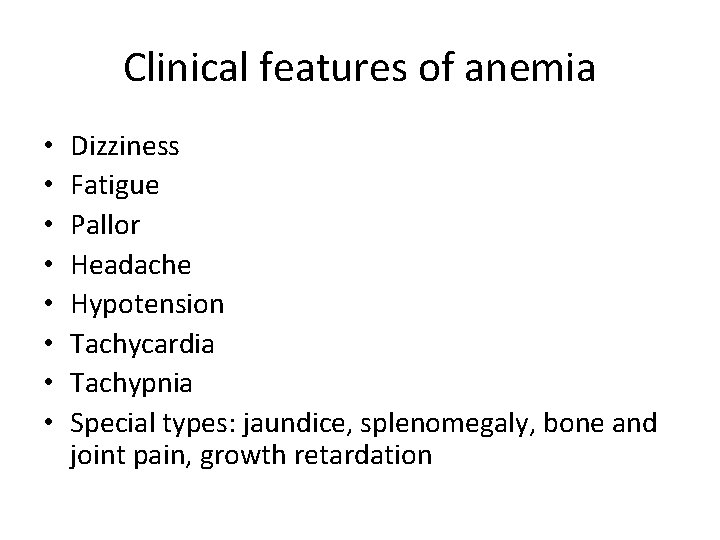
Clinical features of anemia • • Dizziness Fatigue Pallor Headache Hypotension Tachycardia Tachypnia Special types: jaundice, splenomegaly, bone and joint pain, growth retardation
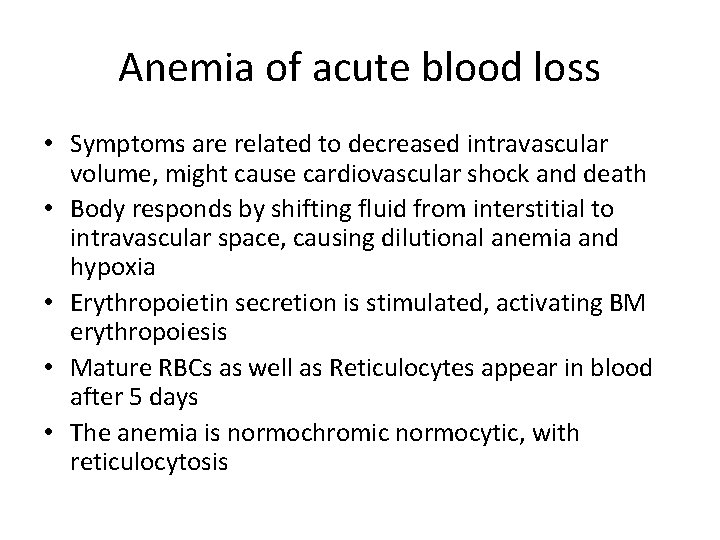
Anemia of acute blood loss • Symptoms are related to decreased intravascular volume, might cause cardiovascular shock and death • Body responds by shifting fluid from interstitial to intravascular space, causing dilutional anemia and hypoxia • Erythropoietin secretion is stimulated, activating BM erythropoiesis • Mature RBCs as well as Reticulocytes appear in blood after 5 days • The anemia is normochromic normocytic, with reticulocytosis
Anemia of chronic blood loss • Occurs when the rate of RBC loss exceeds regeneration • Usually occurs in GI and gynecologic diseases • Body loses iron in lost RBCs, resulting in iron deficiency anemia lately • Anemia begins normochromic normocytic, then hypochromic microcytic
Hemolytic Anemia • Normally, RBCs age is around 120 days, aged RBCs are engulfed by phagocytic cells in spleen • In Hemolytic anemia; premature destruction of RBCs • Accumulation of Hg degradation products (bilirubin) • Secondary increased erythropoiesis • High reticulocyte count • High Lactate Dehydrogenase (LDH) enzyme in serum

Extravascular Hemolysis • Generally caused when the RBC is less deformable or having abnormal shape • Abnormal RBC shape prevents its normal movement in splenic sinusoids • Prolonged time of RBCs passage attracts histiocytes to engulf them • Hg within phagocytes is converted to bilirubin • The triad of extravascular HA is: Anemia, splenomegaly and jaundice

Intravascular Hemolysis • Less common • Caused by mechanical damage, complement fixation, microorganism, exogenous toxins • Free Hg and met Hg are excreted in urine (hemoglobinuria) causing dark urine • Free iron deposits in kidneys resulting in renal hemosiderosis
Hereditary Spherocytosis • This inherited disorder is caused by intrinsic defects in the vertical proteins in red cell membrane skeleton • AD inheritance pattern • Common in Scandinavia
Pathobiology • Deficiency in one of the proteins: α-spectrin, β-spectrin, ankyrin, band 4. 2, or band 3 • Red cells lose membrane fragments and become spheres • Spherocytic cells are less deformable than normal ones and therefore become trapped in the splenic cords, where they are phagocytosed by macrophages • RBC Life span is dropped to less than 20 days

Clinical features • Patients have splenomegaly, extravascular hemolysis signs, family history of anemia and splenectomy • Treatment: splenectomy
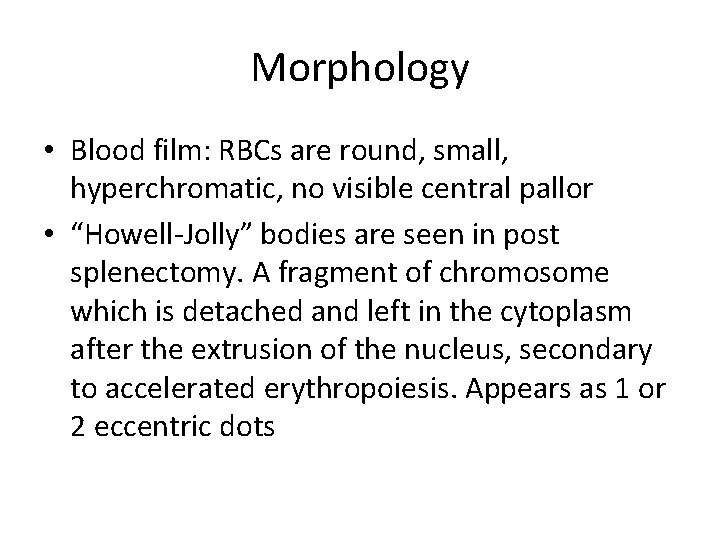
Morphology • Blood film: RBCs are round, small, hyperchromatic, no visible central pallor • “Howell-Jolly” bodies are seen in post splenectomy. A fragment of chromosome which is detached and left in the cytoplasm after the extrusion of the nucleus, secondary to accelerated erythropoiesis. Appears as 1 or 2 eccentric dots
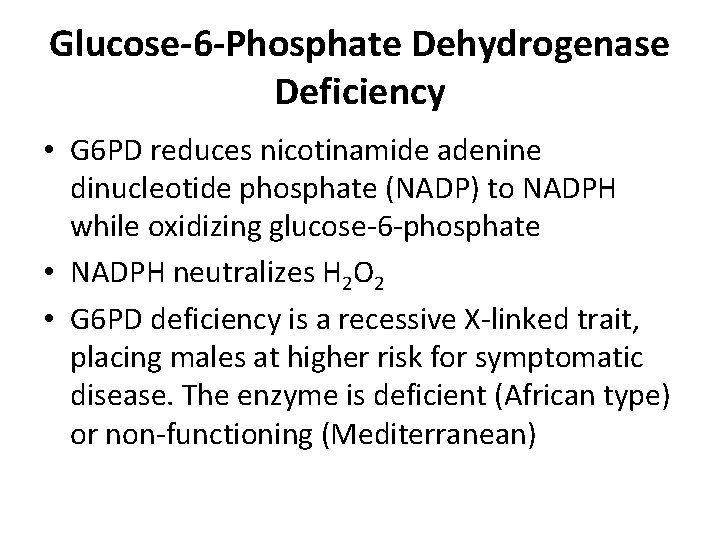
Glucose-6 -Phosphate Dehydrogenase Deficiency • G 6 PD reduces nicotinamide adenine dinucleotide phosphate (NADP) to NADPH while oxidizing glucose-6 -phosphate • NADPH neutralizes H 2 O 2 • G 6 PD deficiency is a recessive X-linked trait, placing males at higher risk for symptomatic disease. The enzyme is deficient (African type) or non-functioning (Mediterranean)
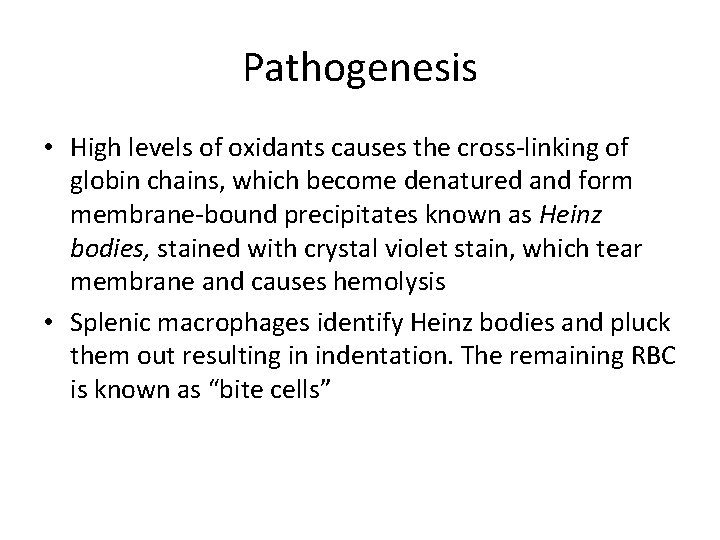
Pathogenesis • High levels of oxidants causes the cross-linking of globin chains, which become denatured and form membrane-bound precipitates known as Heinz bodies, stained with crystal violet stain, which tear membrane and causes hemolysis • Splenic macrophages identify Heinz bodies and pluck them out resulting in indentation. The remaining RBC is known as “bite cells”

Causes of hemolytic crisis • Hemolysis happens upon exposure to oxidant stress • The most common triggers are: • (1) infections, in which oxygen-derived free radicals are produced by activated leukocytes • (2) Drugs (anti malaria, sulfonamides) • (3) Fava beans (favism) • Hemolysis occurs 2 -3 days post exposure, appears as sudden drop in Hg level with pain

Thalassemia • The thalassemia syndromes are a heterogeneous group of disorders caused by inherited mutations that decrease the synthesis of adult hemoglobin, Hg. A (α 2β 2) • Endemic in Middle East, tropical Africa, India, Asia • β-Thalassemia is caused by deficient synthesis of β chains, whereas α-thalassemia is caused by deficient synthesis of α chains
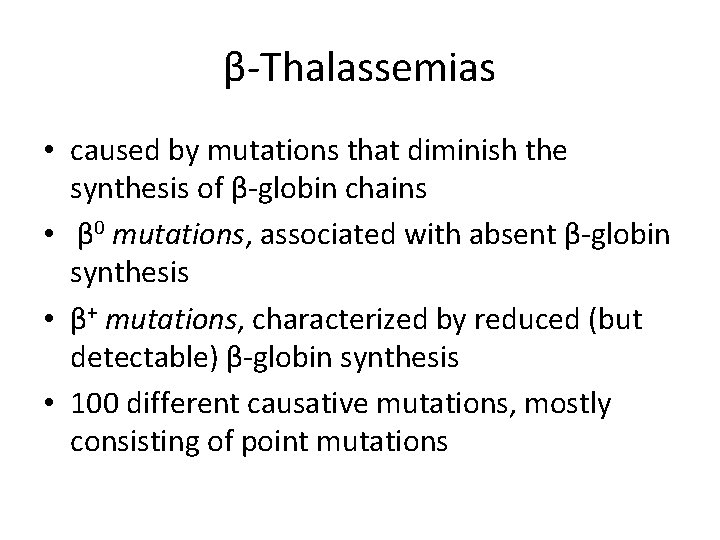
β-Thalassemias • caused by mutations that diminish the synthesis of β-globin chains • β 0 mutations, associated with absent β-globin synthesis • β+ mutations, characterized by reduced (but detectable) β-globin synthesis • 100 different causative mutations, mostly consisting of point mutations
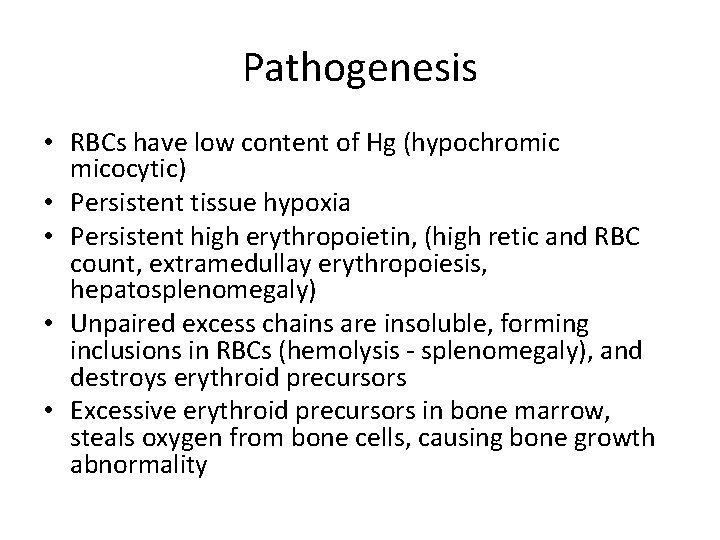
Pathogenesis • RBCs have low content of Hg (hypochromic micocytic) • Persistent tissue hypoxia • Persistent high erythropoietin, (high retic and RBC count, extramedullay erythropoiesis, hepatosplenomegaly) • Unpaired excess chains are insoluble, forming inclusions in RBCs (hemolysis - splenomegaly), and destroys erythroid precursors • Excessive erythroid precursors in bone marrow, steals oxygen from bone cells, causing bone growth abnormality
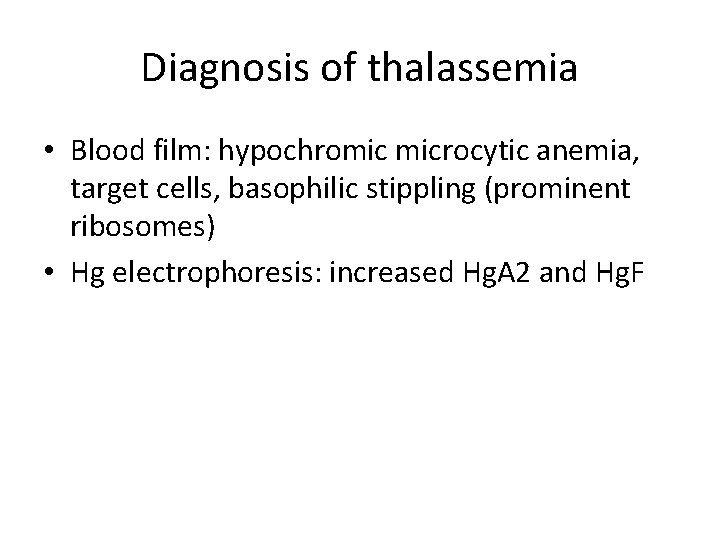
Diagnosis of thalassemia • Blood film: hypochromic microcytic anemia, target cells, basophilic stippling (prominent ribosomes) • Hg electrophoresis: increased Hg. A 2 and Hg. F

Sickle Cell Anemia • Common in Africa and middle east • AR inheritance • Sickle cell disease is caused by a point mutation in the sixth codon of β-globin that leads to the replacement of a glutamate residue with a valine residue • The abnormal physiochemical properties of the resulting sickle hemoglobin (Hb. S) are responsible for the disease

• Sickle Cell Trait: heterozygosity of Hg. S, carriers are largely asymptomatic, Hg. S ≈ 40% • Sickle Cell Disease: homozygosity of Hb. S, symptomatic, Hg. S ≈ 80% • Both types of Hg are protective against Malaria falciparum infection
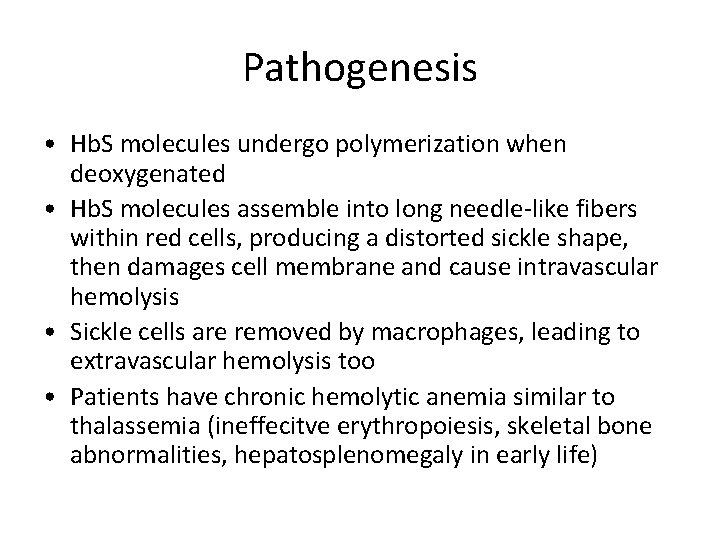
Pathogenesis • Hb. S molecules undergo polymerization when deoxygenated • Hb. S molecules assemble into long needle-like fibers within red cells, producing a distorted sickle shape, then damages cell membrane and cause intravascular hemolysis • Sickle cells are removed by macrophages, leading to extravascular hemolysis too • Patients have chronic hemolytic anemia similar to thalassemia (ineffecitve erythropoiesis, skeletal bone abnormalities, hepatosplenomegaly in early life)
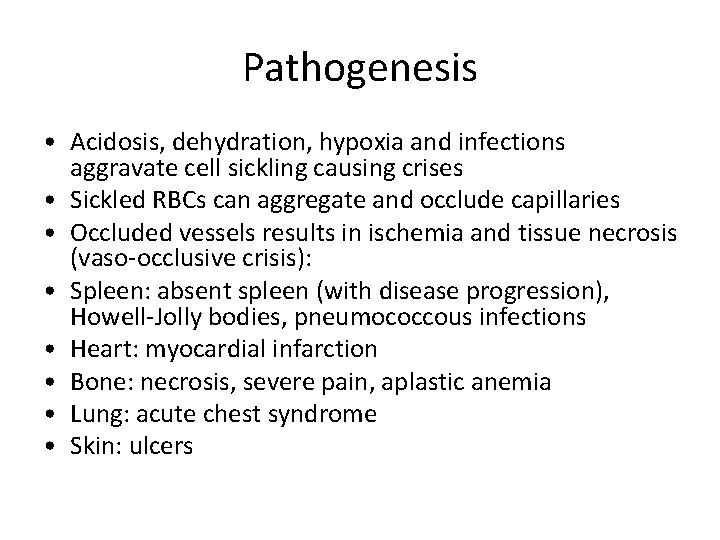
Pathogenesis • Acidosis, dehydration, hypoxia and infections aggravate cell sickling causing crises • Sickled RBCs can aggregate and occlude capillaries • Occluded vessels results in ischemia and tissue necrosis (vaso-occlusive crisis): • Spleen: absent spleen (with disease progression), Howell-Jolly bodies, pneumococcous infections • Heart: myocardial infarction • Bone: necrosis, severe pain, aplastic anemia • Lung: acute chest syndrome • Skin: ulcers
Diagnosis • Blood film: (sickle cells) • Sickling test: application of oxygen-consuming reagent to blood • Hemoglobin electrophoresis is also used to demonstrate the presence of Hb. S
ANEMIAS OF DIMINISHED ERYTHROPOIESIS Anemias secondary to inadequate RBC production • Nutritional • Renal failure • Chronic inflammation • Bone marrow failure

Iron deficiency anemia • The most common anemia worldwide • Nutritional or blood loss • People at increased risk of anemia are: infants, elderly, teenagers, low socioeconomic class • Iron is stored as ferritin in the bone marrow • IDA develops insidiously, begins with decreased stored ferritin, and lastly the serum iron is decreased

• RBCs appear as microcytic and hypochromic, Target cells • Response to iron therapy
Megaloblastic anemia • Anemia associated with impairment in DNA synthesis in hematopoietic cells special morphologic features (large immature erythroid precursors) • Two types: Vitamin B 12 and folate deficiency • Vitamin B 12 and folate are coenzymes required for synthesis of thymidine • Vitamin B 12 is essential for myelin synthesis, deficiency causes anemia and neurologic disease

Causes of Vit B 12 deficiency • Low intake (vegans) • Impaired GI absorption (pernicous anemia, malabsorption disease, gastrectomy) • Pernicious anemia: autoimmune disease, destruction to parietal cells, impaired absorption

Causes of folate deficiency • Low intake (inadequate diet, infancy) • Impaired absorption (malabsorption, chronic alcoholism, anti-convulsants, oral contraceptives) • Impaired utilization (methotrexate, Vit B 12 deficiency) • Increased demand: pregnancy

Morphology • PB: RBCs are large and oval and no central pallor • Reticulocytes are low • Neutrophils are large and have hypersegmented nuclear lobes (5 or more) • BM: Megaloblastic changes in erythroid precursors (large size and immature nucleus despite cytoplasmic maturation)
Anemia of Chronic Disease • Most common anemia in hospitalized people • Occurs in chronic inflammatory diseases (infection, autoimmune, cancer) • IL-6 activates the synthesis of Hepcidin in the liver, which suppresses erythropoietin and prevents the transfer of iron to erythroid cells • Anemia is normochromic normocytic, or hypochromic microcytic

Aplastic Anemia • Bone marrow fail to produce the 3 cell lines • 60% of cases are idiopathic. The rest are secondary to drugs (chloramphenicol), chemicals (benzene), infection (hepaitits) or autoimmune process
POLYCYTHEMIA • increase in hemoglobin concentration and hematochrit • Primary polycythemia (polycythemia vera) is a clonal, neoplastic myeloproliferative disorder • Secondary polycythemia occurs as an Adaptive process (lung disease, high-altitude living, cyanotic heart disease), Paraneoplastic: erythropoietin-secreting tumors (e. g. , renal cell carcinoma), or Surreptitious: endurance athletes

White blood cell disorders

Leukopenia • Neutropenia: occurs as part of aplastic anemia, drug reaction (anti-epileptic, antithyroid, chemotherapy), congenital. Patients develop severe bacterial infections • Lymphopenia is much less common; it is associated with congenital immunodeficiency diseases, advanced human immunodeficiency virus (HIV) infection, and treatment with high doses of corticosteroids

Reactive Leukocytosis • An increase in the number of white cells in the blood is common in a variety of inflammatory states caused by microbial and nonmicrobial stimuli
Neutrophilia • Infection (bacterial) • Burn • Tissue necrosis (myocardial infarction)

Eosinophilia • Allergic reactions • Parasitic infections • Drug reactions
Lymphocytosis • Viral infections • Tuberculosis
Reactive Lymphadenitis • Any immune response against foreign antigens can lead to lymph node enlargement (lymphadenopathy) Acute lymphadenitis: neutrophilic infiltration (bacterial), painful Chronic lymphadenitis: • B-cell proliferation (Follicular hyperplasia): seen in HIV, rheumatologic diseases and toxoplasmosis • T-cell proliferation (Paracortical hyperplasia): caused by viral infection, drug reaction, post vaccination

Lymphoma • Generally classified as Hodgkin and non-Hodgkin lymphomas • Non-Hodgkin lymphoma is classified as B or T-cell lymphoma, which both are further classified as low or high grade • Most lymphomas arise in lymph nodes, however, they can arise from any organ, called: extranodal lymphomas • B-cell lymphomas express CD 20 • T-cell lymphomas express CD 3 • Hodgkin lymphomas express CD 30 • Patients with lymphoma have disturbed immune system
Follicular Lymphoma Common, low-grade B-cell lymphoma Affects elderly Arises from germinal center B-cell Lymphoma cells have specific translocation t(14: 18), in which Bcl 2 gene on chr 18 fuses with Ig. H gene on chr 14, causing overexpression of Bcl 2 • Patients has generalized lymphadenopathy • Lymphoma cells proliferate to form abnormal, large, crowded follicles • Patients have indolent course, transforms into high grade lymphoma in 40% of cases • •
Diffuse Large B Cell Lymphoma • most common type of lymphoma in adults, accounting for approximately 50% of adult NHLs, • Arises de novo, as a transformation from low grade B-cell lymphoma, in the setting of chronic immune stimulation • High-grade lymphoma, progressive and fatal if not treated
Chronic Lymphocytic Leukemia • Low grade B-cell lymphoma • Cells are small, round, mature looking similar to normal lymphocytes • Affects BM and blood (CLL), or LN (SLL) • Bcl 2 (anti-apoptotic protein) is up-regulated • The most common leukemia in elderly • Indolent course, stays stable for years • 10% transforms into high-grade lymphoma

Hodgkin Lymphoma • a group of lymphoid neoplasms that differ from NHL in several respects, common in children and young adults • More often localized to a single axial group of nodes (cervical, mediastinal, axillary) • Orderly spread by contiguity • Characterized by the presence of Reed-Sternberg cells (abnormal B-cell in origin but does not resemble or express the normal B-cell markers)
Acute Lymphoblastic Leukemia • An aggressive, high-grade type of lymphoma • Arises from precursor lymphoid cells (lymphoblasts), B or T • B-ALL is the most common cancer is children, arises from BM • T-ALL occurs mainly in male adolescents, arises from thymus • Lymphoblasts develop mutations in transcription genes which regulate both lymphocyte differentiation and proliferation • Patients present with sudden fever (infection), bleeding and anemia
Acute myeloid leukemia • Arises from myeloblasts in bone marrow, which differentiate into the 3 cell lines • Caused by acquired mutations that impede differentiation, and increases proliferation, leading to the accumulation of immature myeloid blasts in the marrow • Bone marrow become filled with blasts, which destroys the normal cells and cause bone marrow failure • Myeloblasts circulate in the blood (leukemia) • Affects all age groups
Myelodysplastic syndrome • Pre-leukemic stage • Acquired genetic mutations in the myeloblasts, which inhibit the normal maturation • Patients develop refractory anemia, thrombocytopenia and neutropenia • Myeloblasts are not increased in number • Affects elderly
Chronic Myelogenous Leukemia • A chronic leukemia in which myeloblasts have translocation between chromosomes 9 (ABL gene) and 22 (BCR) • The new chromosome is known as Philadelphia chromosome • The new fusion gene (BCR-ABL) gain a new “Tyrosine-Kinase” function, which activates cell proliferation and prolonged its survival • Bone marrow and blood become filled with myeloid cells of all stages of maturation (from blasts to neutrophils)
- Slides: 53