Rayat Shikshan Sansthas Yashavantrao Chavan Institute of Science











- Slides: 27

Rayat Shikshan Sanstha’s , Yashavantrao Chavan Institute of Science Satara Presentation on SURFACE TENSION Shri. Jadha. V s. k.

Class- XII Physics - I SURFACE TENSION

Surface Tension Surface tension is the property by virtue of which the free surface of a liquid behave like a stretched elastic membrane under tension and tries to have minimum possible area. Examples : 1) Rain drops are spherical in shape due to surface tension 2) Insects can walk on the surface of water due to surface tension

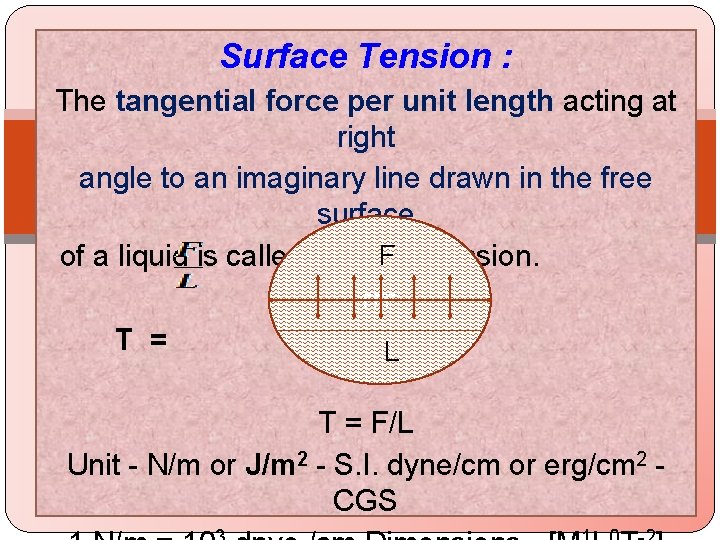
Surface Tension : The tangential force per unit length acting at right angle to an imaginary line drawn in the free surface F of a liquid is called Surface Tension. FFF T = L T = F/L Unit - N/m or J/m 2 - S. I. dyne/cm or erg/cm 2 - CGS

Application 1) The force required to lift a thin wire or needle of length l from the surface of water or liquid is F = T x 2 L 2) Force required to lift a thin rectangular plate from the surface of water is F = 2 T (L + b) where l is length and b is breadth. 3) Force required to lift thin circular plate from the surface of water is F = T x 2π r, where r - radius of the plate. 4) Force required to lift a ring from surface of water is

Intermolecular force : The force of attraction or repulsion between two molecules of substance is called intermolecular force. 1) Cohesive force : The force of attraction between the molecules of two same substance is called as Cohesive force

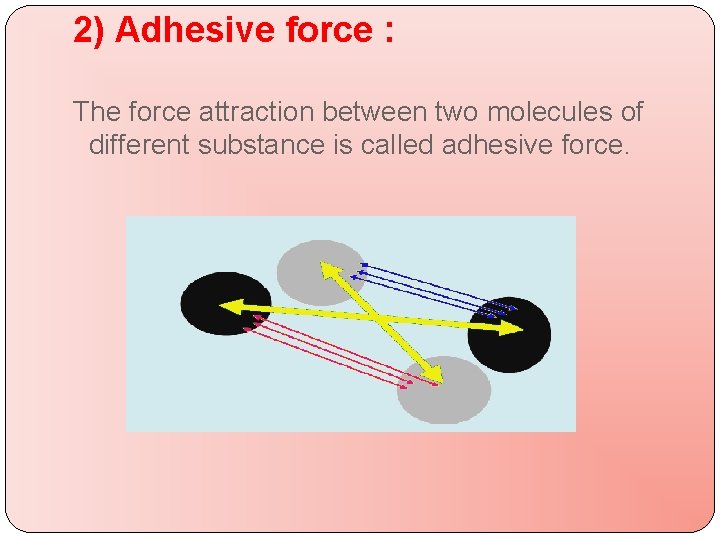
2) Adhesive force : The force attraction between two molecules of different substance is called adhesive force.

Range of Molecular Attraction : The maximum distance up to which the intermolecular force of attraction is effective is called range of molecular attraction. Sphere of influence : An imaginary sphere drawn with a molecule as centre and the range of molecular attraction as radius is called the sphere of influence. Surface film The thin layer of the liquid whose thickness is equal to range of molecular attraction.

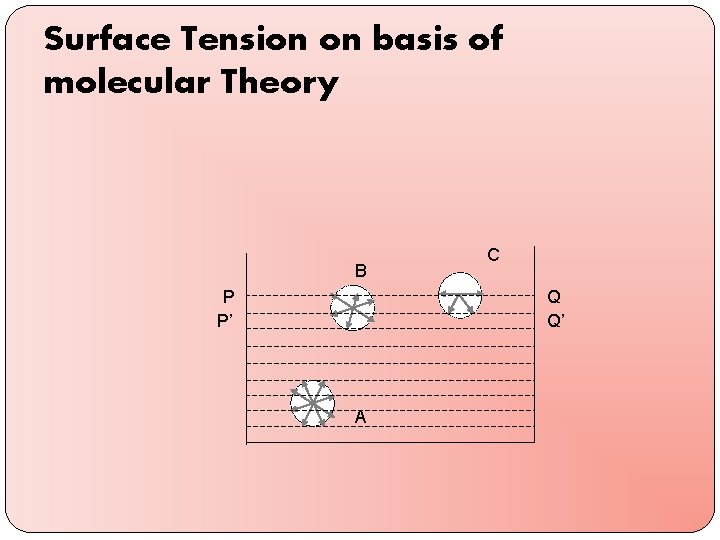
Surface Tension on basis of molecular Theory B P P’ C Q Q’ A

�Surface energy— The potential energy per unit surface area is called surface energy per unit area of surface. � �Units-- S. I. — joule � CGS – erg � �Dimensions— �

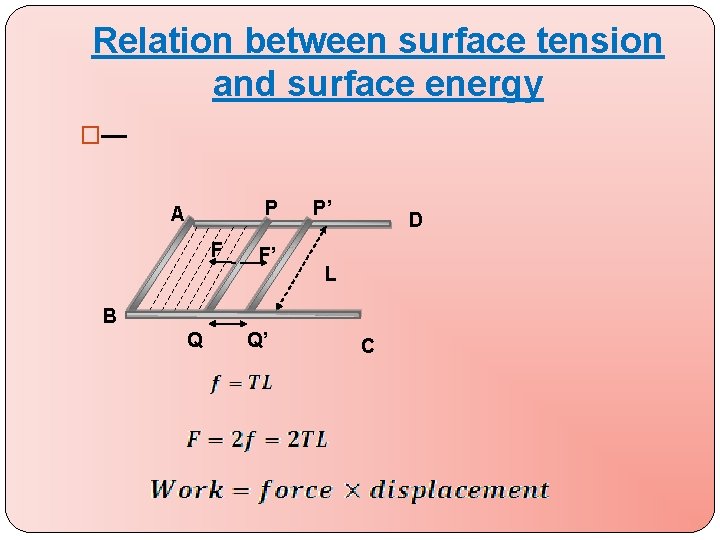
Relation between surface tension and surface energy �— P A F F’ P’ D L B Q Q’ C

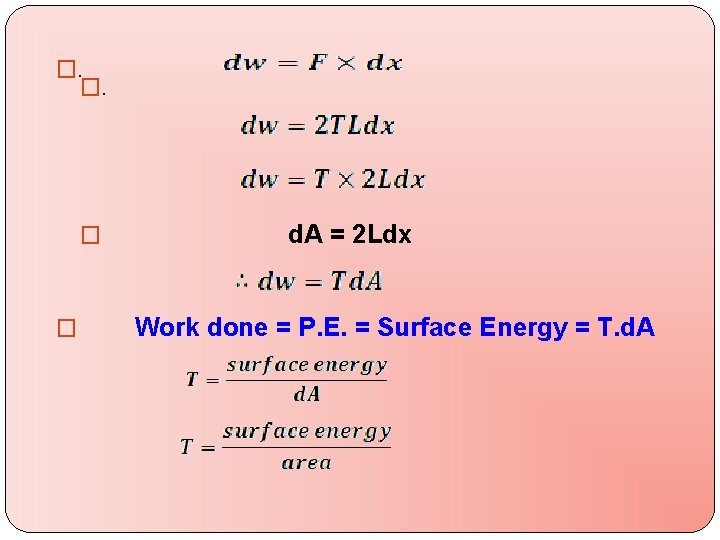
�. �. � � d. A = 2 Ldx Work done = P. E. = Surface Energy = T. d. A

Angle of Contact The angle between the tangent drawn to the liquid surface at point of contact and solid surface measured on the side the liquid is called the angle of contact. (θ) Characteristics of angle of contact : 1. It is constant for a given liquid- solid pair 2. The liquid, which completely wets solid it is zero e. g. pure water in contact with glass. 3. The liquid which partially wets the solid it is acute e. g. Kerosene in contact with glass.

Characteristics of angle of contact : 4) The liquid which does not wet’s the solid, it is obtuse e. g. Mercury in contact with glass. 5) It increases with increasing temperature 6) A water proofing agent (powder) increases the angle of contact. 7) It decreases with adding insoluble or partially soluble impurity. 8) It increases with adding soluble impurity. 9) It depends upon medium above the free surface.

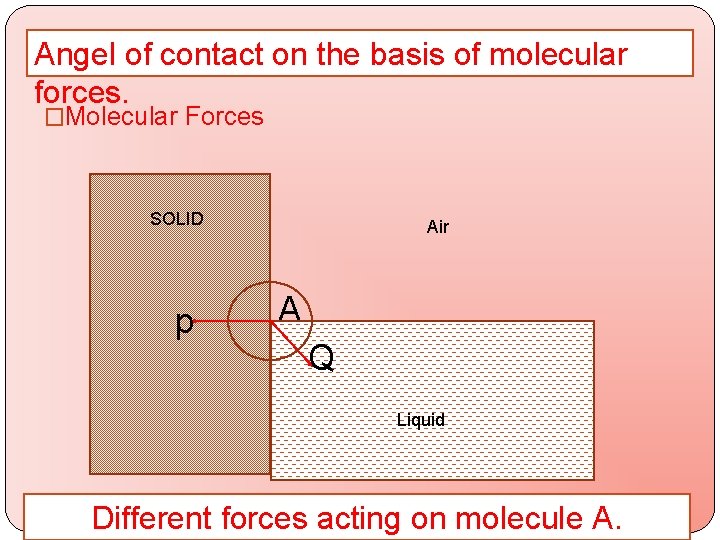
Angel of contact on the basis of molecular forces. �Molecular Forces SOLID p Air A Q Q LIQUID Liquid Different forces acting on molecule A.

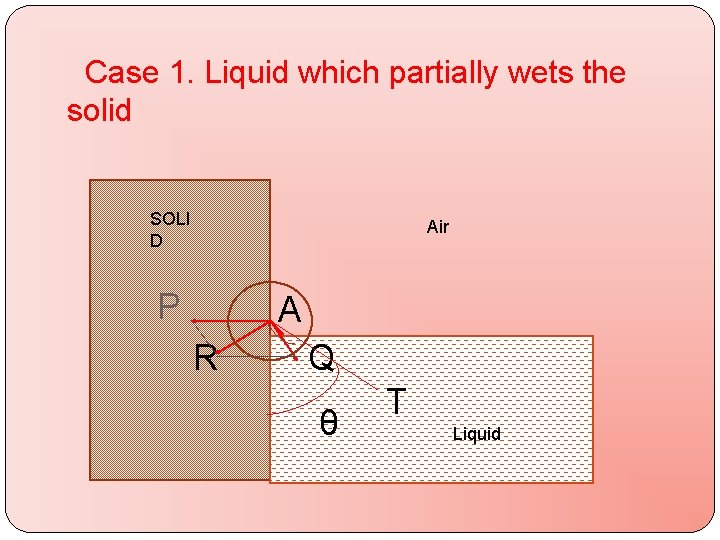
Case 1. Liquid which partially wets the solid SOLI D Air P A R Q Q TLIQUID θ Liquid

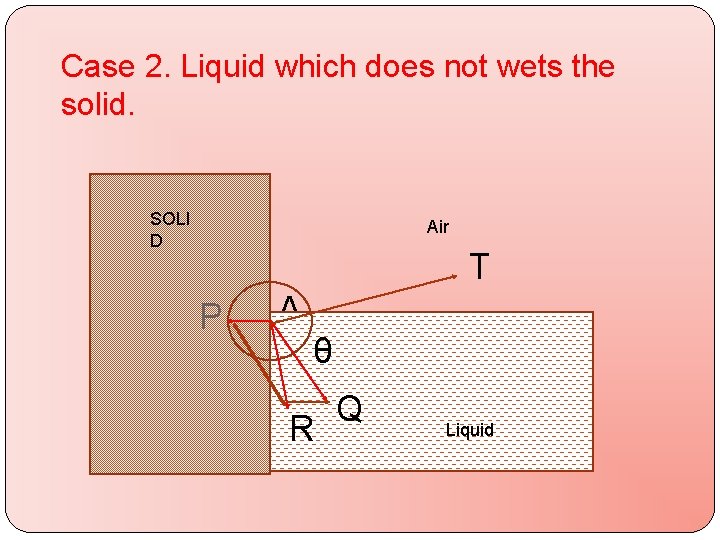
Case 2. Liquid which does not wets the solid. SOLI D Air T P A θ R Q Q LIQUID Liquid

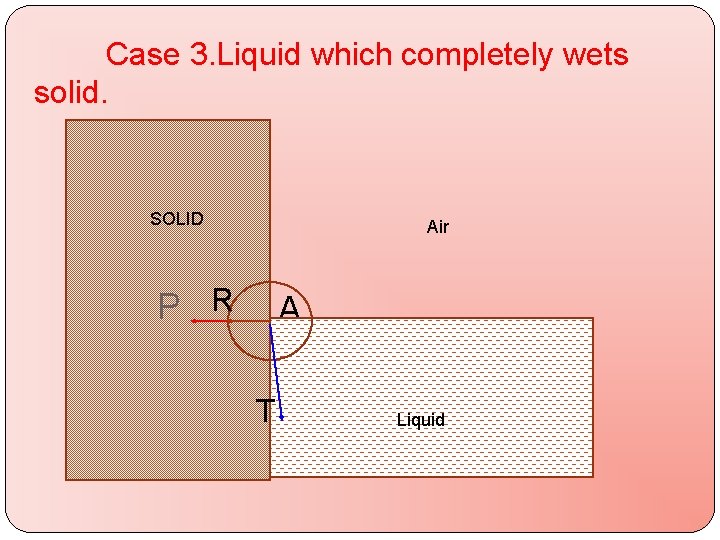
Case 3. Liquid which completely wets solid. SOLID Air P R A T Q LIQUID Liquid

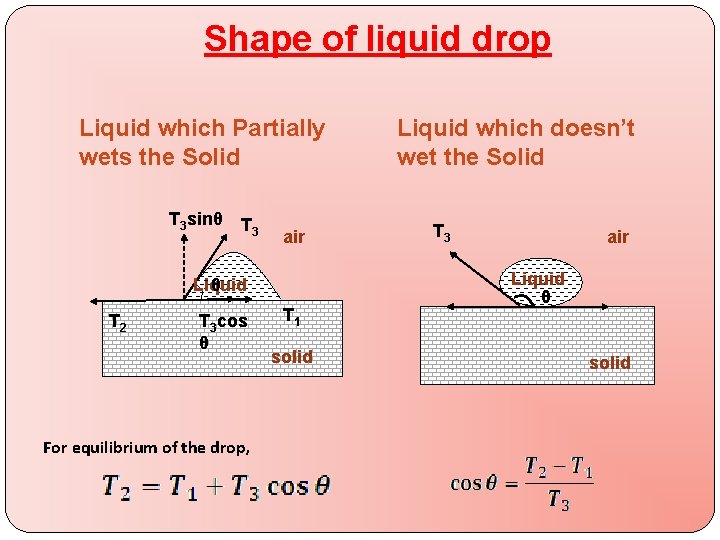
Shape of liquid drop Liquid which Partially wets the Solid T 3 sinθ T 3 air Liquid θ T 2 T 3 cos θ For equilibrium of the drop, T 1 solid Liquid which doesn’t wet the Solid T 3 air Liquid θ solid

This equation gives following cases � Case 1 : � If and � cosθ is positive � θ is acute � ex. kerosene in contact with glass � Case 2 : � If � cosθ is negative � θ is obtuse � ex. mercury in contact with glass � Case 3 : � If � cosθ = 1 � θ is nearly equal to zero � Ex. water in contact with glass � Case 4 : � If or � cosθ > 1 � which is impossible � liquid is spread over the solid surface and drop shall not be formed.

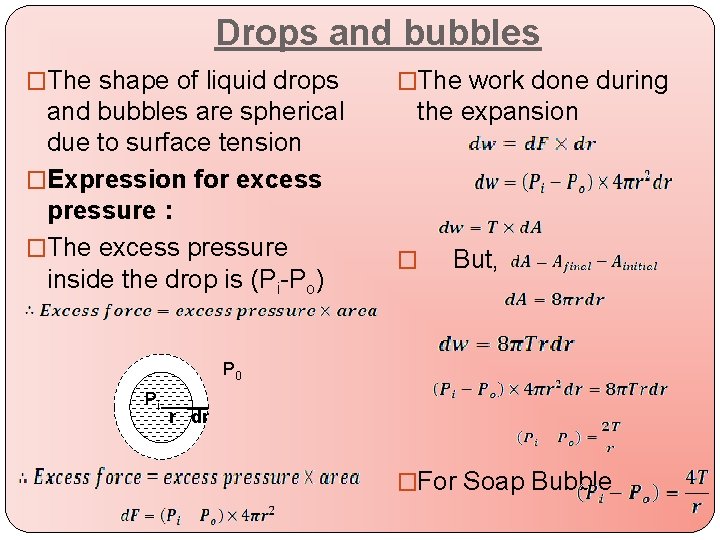
Drops and bubbles �The shape of liquid drops and bubbles are spherical due to surface tension �Expression for excess pressure : �The excess pressure inside the drop is (Pi-Po) �The work done during the expansion � But, P 0 Pi r dr �For Soap Bubble

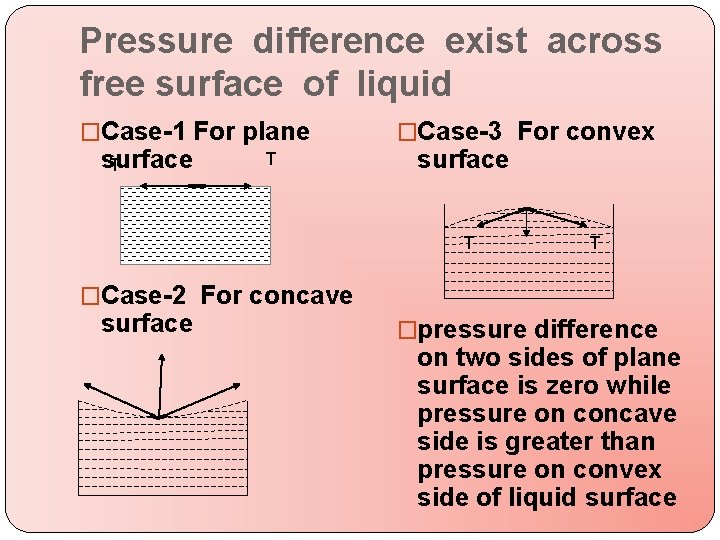
Pressure difference exist across free surface of liquid �Case-1 For plane surface T T �Case-3 For convex surface T T �Case-2 For concave surface �pressure difference on two sides of plane surface is zero while pressure on concave side is greater than pressure on convex side of liquid surface

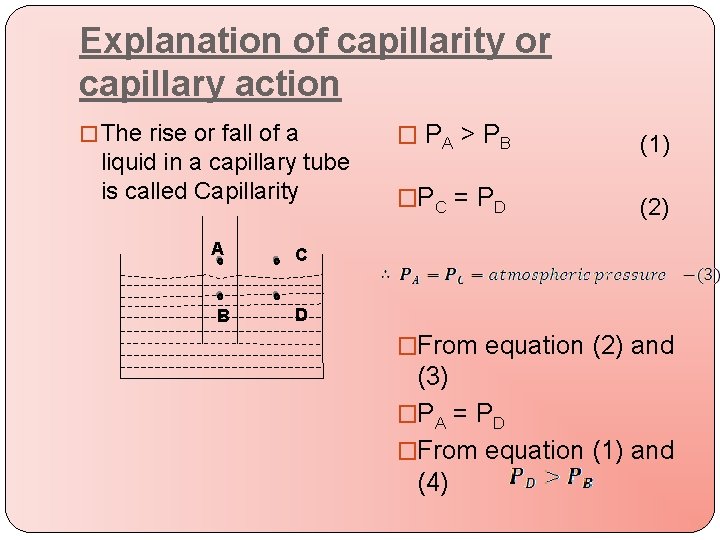
Explanation of capillarity or capillary action � The rise or fall of a liquid in a capillary tube is called Capillarity A C B D � PA > PB (1) �PC = PD (2) �From equation (2) and (3) �PA = PD �From equation (1) and (4)

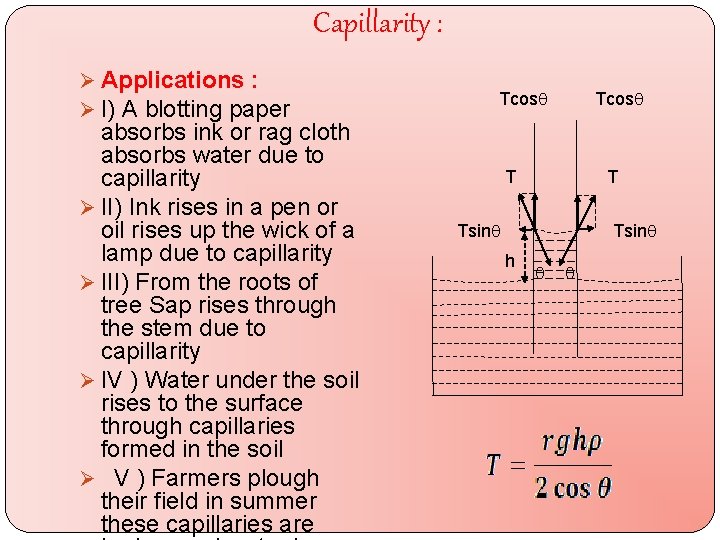
Capillarity : Ø Applications : Ø I) A blotting paper absorbs ink or rag cloth absorbs water due to capillarity Ø II) Ink rises in a pen or oil rises up the wick of a lamp due to capillarity Ø III) From the roots of tree Sap rises through the stem due to capillarity Ø IV ) Water under the soil rises to the surface through capillaries formed in the soil Ø V ) Farmers plough their field in summer these capillaries are Tcos T T Tsin h

Factor’s affecting on Surface tension 1) Effect of temperature on S. T. : The surface tension decreases with increasing temperature. In some exceptional cases the S. T. of the liquid increases with increasing temperature. e. g. molten Cu & Cd Application : It is easier to wash clothes in hot water than ordinary water because S. T. of hot water is less than cold water which help to clean clothes

2) Effect of impurity on Surface tension �i) S. T increases with highly Soluble impurity. � e. g. Na. Cl ii )S. T decreases with weakly or insoluble impurity. Application � a) Soap or any detergents are used for washing clothes purposes because they reduces S. T. and help to remove dust particles from dirty clothes �b) Tooth paste used for cleaning the teeth which reduces S. T. and helps cleaning teeth

�. . Thank You
 Rayat shikshan sanstha sadhana vidyalaya hadapsar
Rayat shikshan sanstha sadhana vidyalaya hadapsar Sadhana vidyalaya hadapsar
Sadhana vidyalaya hadapsar Rayat shikshan sanstha kamothe
Rayat shikshan sanstha kamothe Rayat shikshan sanstha sadhana vidyalaya hadapsar
Rayat shikshan sanstha sadhana vidyalaya hadapsar Rayat shikshan sanstha loni
Rayat shikshan sanstha loni Rayat shikshan sanstha motto is
Rayat shikshan sanstha motto is Rose rayat
Rose rayat Apollonius theorem
Apollonius theorem Rayat shikshan sanstha motto
Rayat shikshan sanstha motto Yashwantrao chavan college
Yashwantrao chavan college उद्दीपकाचे
उद्दीपकाचे Smjoshi.rayat.dc
Smjoshi.rayat.dc Smjoshi.rayat.dc
Smjoshi.rayat.dc Smjoshi.rayat.dc
Smjoshi.rayat.dc Sing kepanjing prosa rayat yaiku….
Sing kepanjing prosa rayat yaiku…. Smjoshi.rayat.dc
Smjoshi.rayat.dc Smjoshi.rayat.dc
Smjoshi.rayat.dc Mpasc.rayat.dc
Mpasc.rayat.dc Smjoshi.rayat.dc
Smjoshi.rayat.dc Your favorite subject
Your favorite subject Ramraj classes nashik
Ramraj classes nashik Ulsan university of science and technology
Ulsan university of science and technology Institute of industrial science the university of tokyo
Institute of industrial science the university of tokyo Masdar institute of science and technology
Masdar institute of science and technology Madhav institute of technology and science
Madhav institute of technology and science Scrambler science olympiad
Scrambler science olympiad Korea institute of sport science
Korea institute of sport science Schorarly
Schorarly















































