Rayat shikshan sansthas th Class10 TEACHERS NAME Mrs













- Slides: 34


Rayat shikshan sanstha’s th Class-10 TEACHERS NAME- Mrs. Manisha Vikas Nikam M. sc. B. Ed.

Science School of elements

SCHOOL OF ELEMENTS

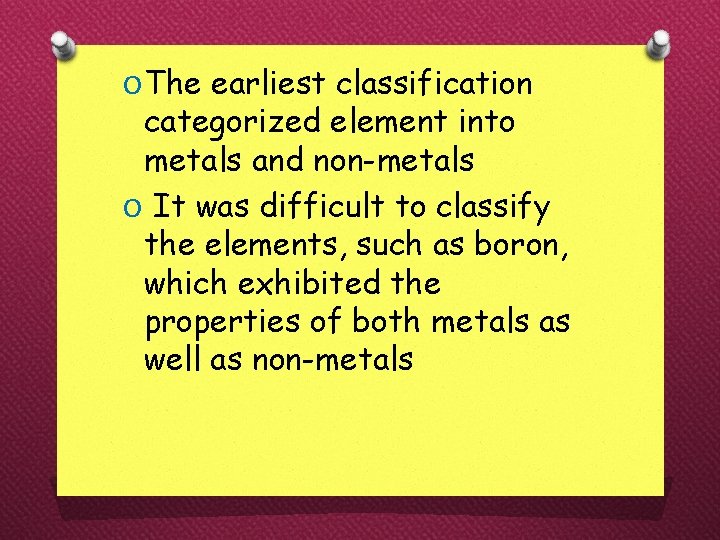
O The earliest classification categorized element into metals and non-metals O It was difficult to classify the elements, such as boron, which exhibited the properties of both metals as well as non-metals

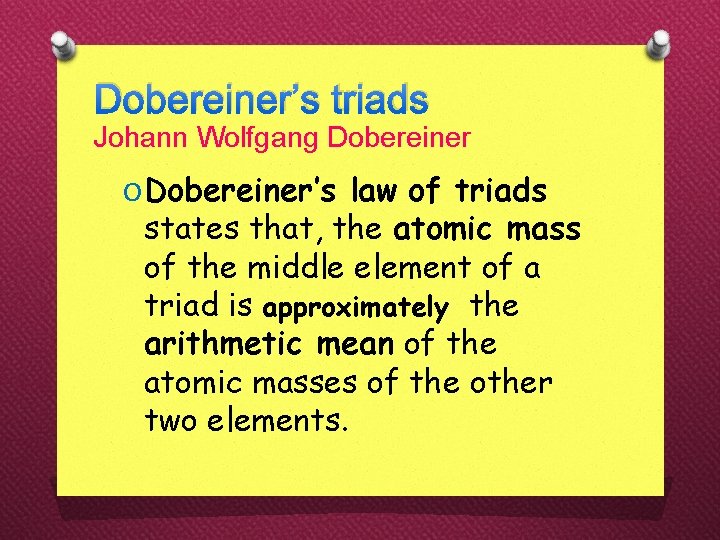
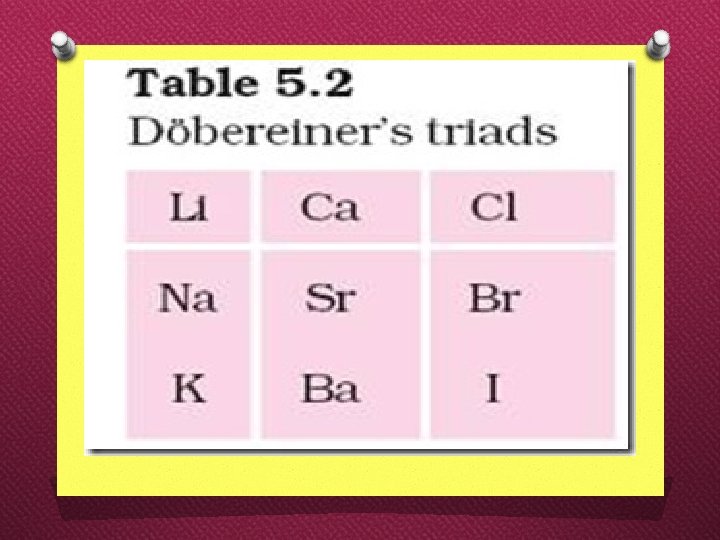
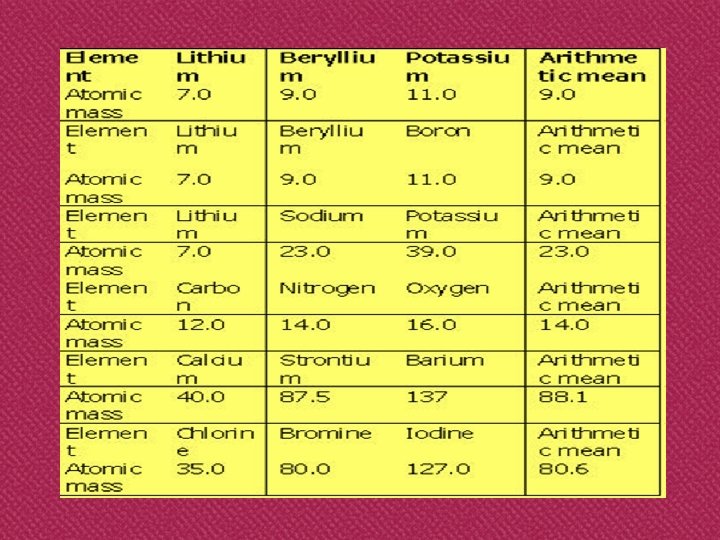
Dobereiner’s triads Johann Wolfgang Dobereiner O Dobereiner’s law of triads states that, the atomic mass of the middle element of a triad is approximately the arithmetic mean of the atomic masses of the other two elements.


Dobereiner’s

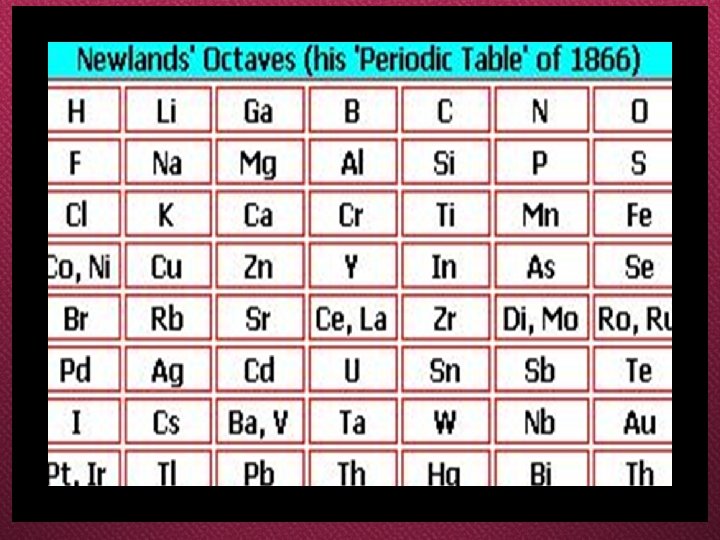
Newland

Newlands’ Law of Octaves O When the elements are arranged in an increasing order of their atomic masses , every eighth element has properties similar to that of the first element,


Some Features of Newlands’ Periodic Table Newlands’ law of octaves is applicable only up to calcium out of total 56 elements known at that time. ii. After calcium every eighth element did not possess properties similar to that of the first. iii. It was assumed by Newlands that only 56 elements existed. But later several elements were discovered. i.

iv. . To fit the existing elements Newlands adjusted two elements in the same slot which differed in their properties. v. Newlands periodic table did not include noble (inert) gases because they were not known at that time.

Dmitri Mendeleev (1834 -1907)

Mendeleev’s periodic law �Mendeleev’s periodic law states that the physical and chemical properties of all elements are a periodic function of their atomic masses. �Atomic weight and chemical reactivity are the two parameters he choose for classifying the elements.

Main features of Mendeleev’s periodic table O The table had 8 vertical columns called groups, and 7 horizontal rows called periods. O In every group, a gradation of physical and chemical properties of elements was observed O The table provided gaps for undiscovered elements

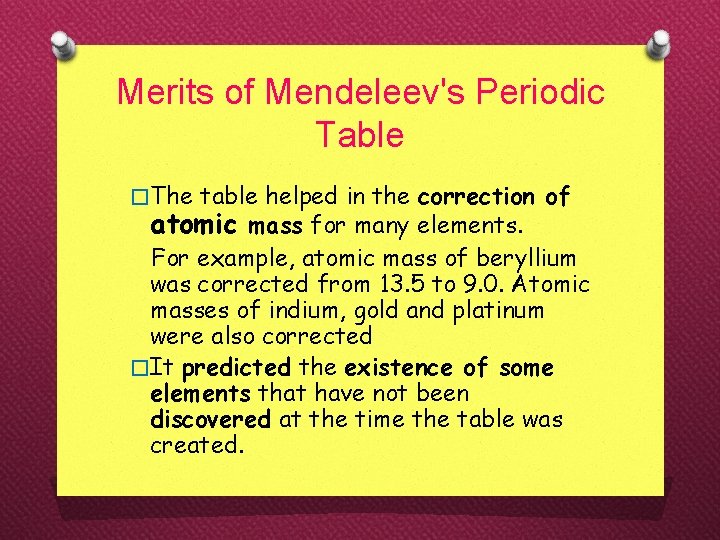
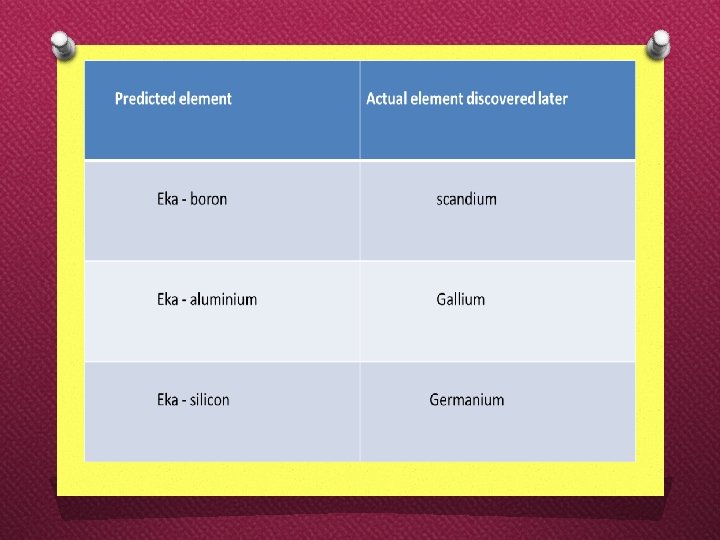
�The table helped predict the properties of three elements. These elements were named ekaboron, eka-aluminium and ekasilicon. �When these elements were discovered, they were named scandium, gallium and germanium.

Merits of Mendeleev's Periodic Table �The table helped in the correction of atomic mass for many elements. For example, atomic mass of beryllium was corrected from 13. 5 to 9. 0. Atomic masses of indium, gold and platinum were also corrected �It predicted the existence of some elements that have not been discovered at the time the table was created.




O Mendeleev's periodic table could accommodate noble gases when they were discovered.

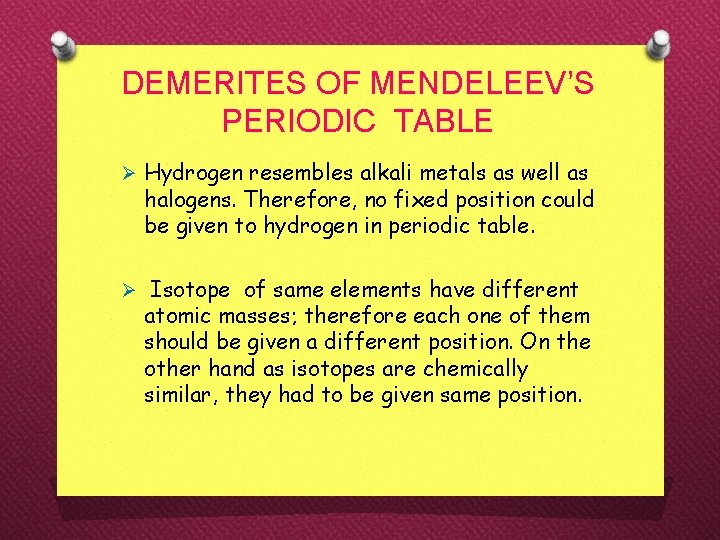
DEMERITES OF MENDELEEV’S PERIODIC TABLE Ø Hydrogen resembles alkali metals as well as halogens. Therefore, no fixed position could be given to hydrogen in periodic table. Ø Isotope of same elements have different atomic masses; therefore each one of them should be given a different position. On the other hand as isotopes are chemically similar, they had to be given same position.

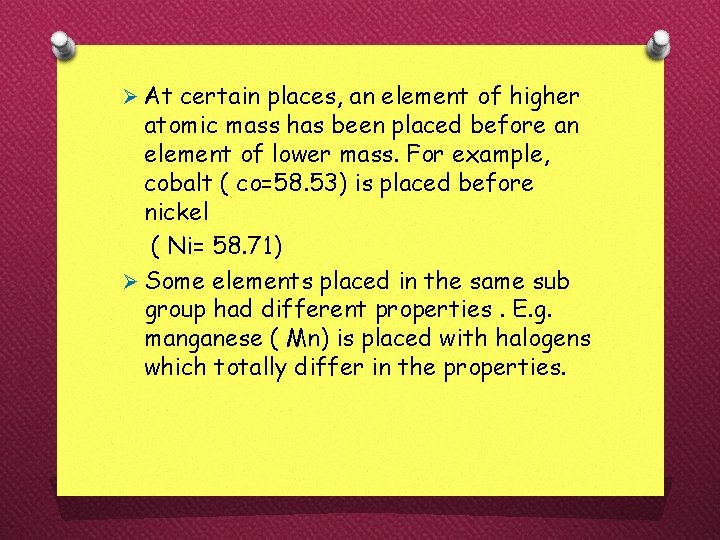
Ø At certain places, an element of higher atomic mass has been placed before an element of lower mass. For example, cobalt ( co=58. 53) is placed before nickel ( Ni= 58. 71) Ø Some elements placed in the same sub group had different properties. E. g. manganese ( Mn) is placed with halogens which totally differ in the properties.


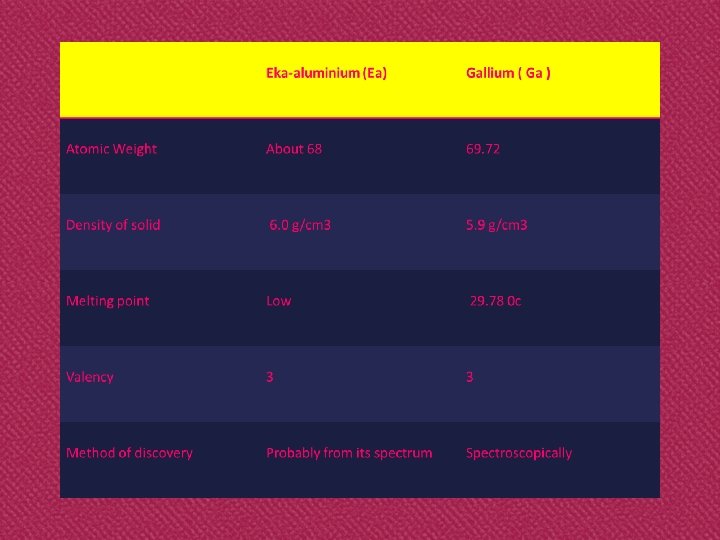
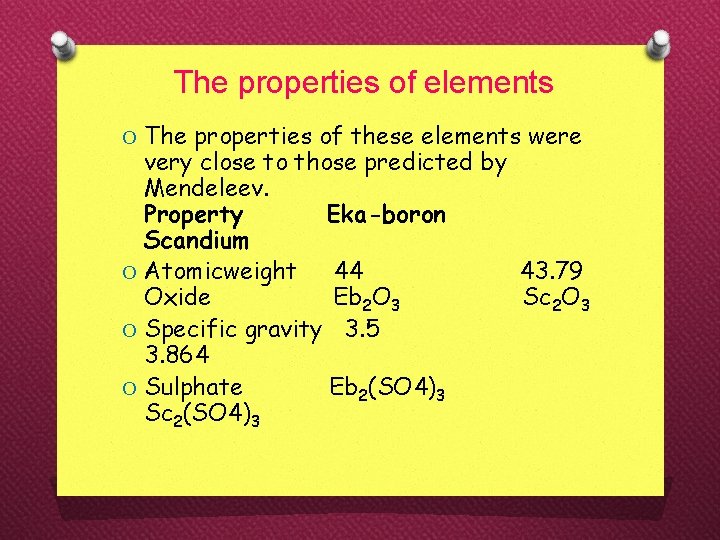
The properties of elements O The properties of these elements were very close to those predicted by Mendeleev. Property Eka-boron Scandium O Atomicweight 44 43. 79 Oxide Eb 2 O 3 Sc 2 O 3 O Specific gravity 3. 5 3. 864 O Sulphate Eb 2(SO 4)3 Sc 2(SO 4)3

Isotopes O Isotopes are atoms of the same element having different atomic mass but same atomic number. O For e. g. , there are three isotopes of hydrogen with atomic mass 1, 2, and 3. O According to Mendeleev's periodic table these should be placed at three separate places. O However isotopes have not been given separate places in the periodic table.


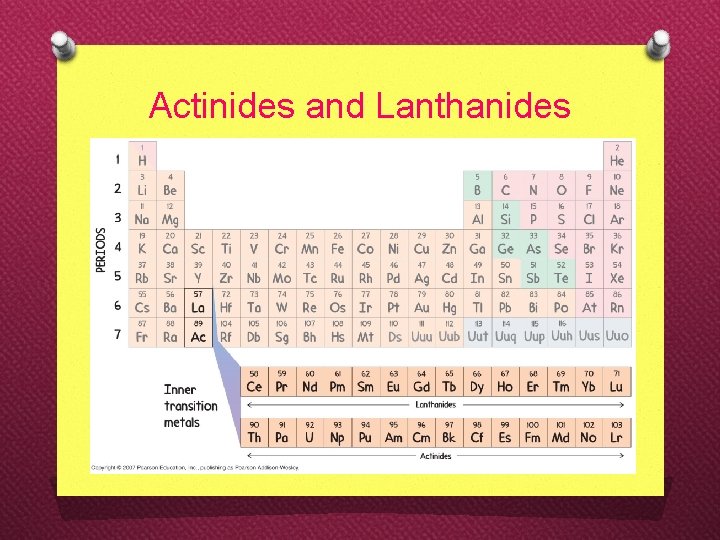
Actinides and Lanthanides

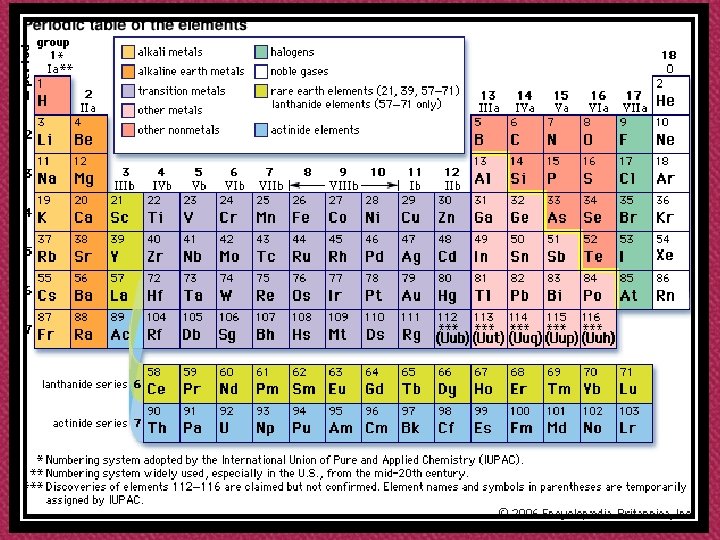
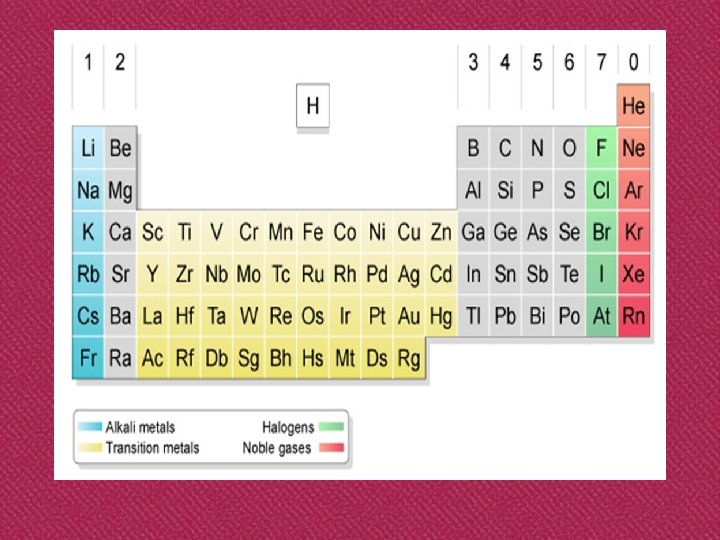
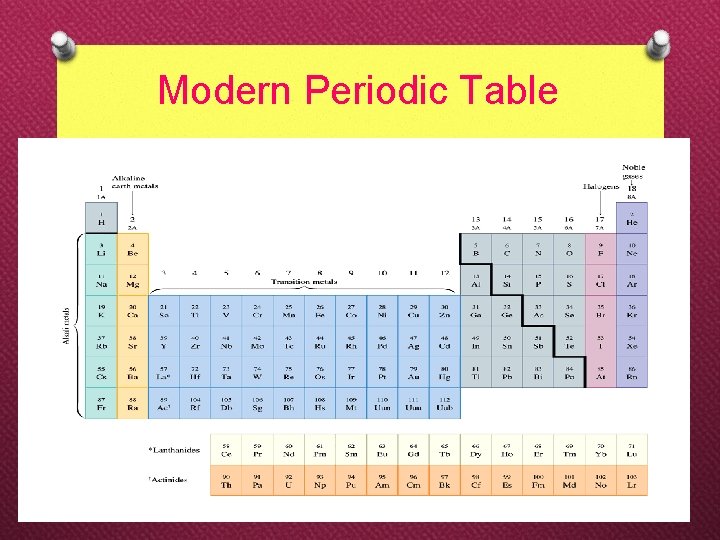
Modern Periodic Table

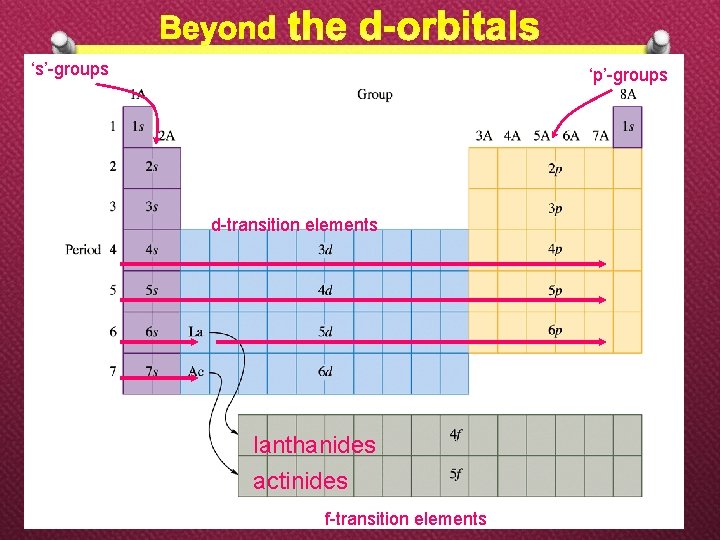
‘s’-groups ‘p’-groups d-transition elements lanthanides actinides f-transition elements

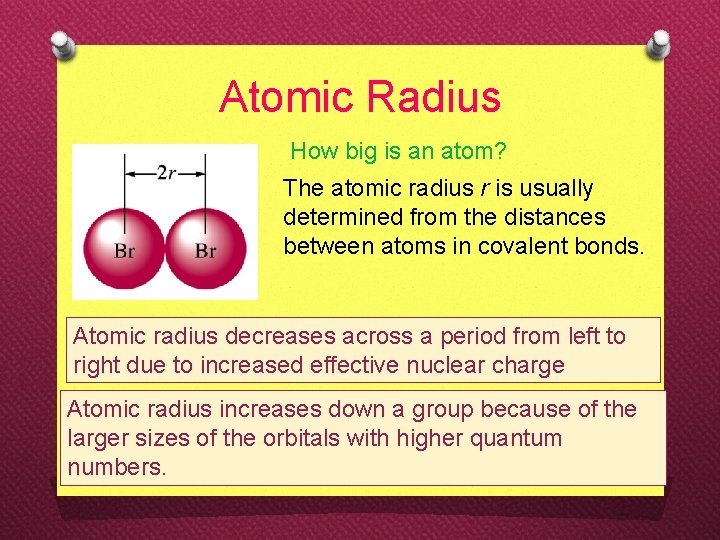
Atomic Radius How big is an atom? The atomic radius r is usually determined from the distances between atoms in covalent bonds. Atomic radius decreases across a period from left to right due to increased effective nuclear charge Atomic radius increases down a group because of the larger sizes of the orbitals with higher quantum numbers.

