Rates of Reactions Measurements Graphs Factors affecting Rate



























- Slides: 55

Rates of Reactions • Measurements & Graphs • Factors affecting Rate of a Reaction • Experiments to show the rate is affected by various factors

What is meant by the rate of a reaction? • A measure of how fast a reaction is taking place. • Different reaction occur at different rates. • E. g. Rusting of iron is slow but reaction of magnesium with dilute hydrochloric acid is fast.

• Reactions involving ions are generally very fast. This relates to the type of bonds present in the reactant particles. • Reactions involving molecular compounds are slow. Why? • The covalent bonds within reactant molecules have to be broken before new bonds are formed between atoms to form products.

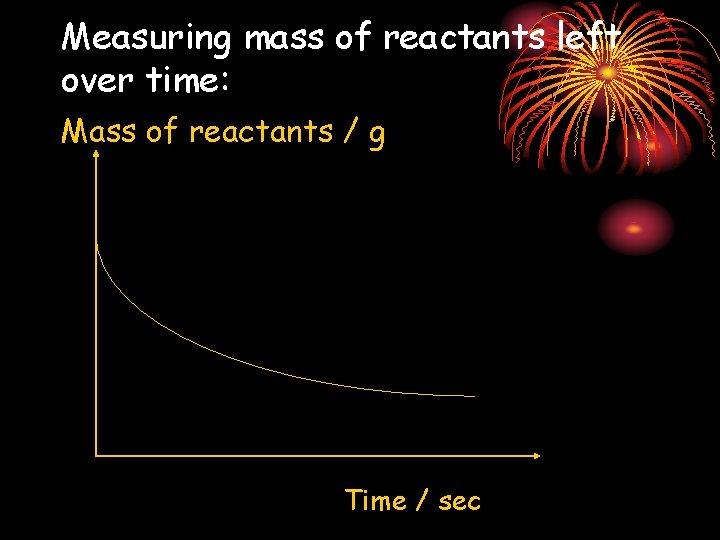
Measurement of the rates of a chemical reaction : • The quantity of products formed over time i. e. volume of gaseous products. • The quantity of the reactants left behind with time i. e. mass of reactants left over time. NOTE that one of the products must be gaseous & is lost to the surroundings. • The time taken for the completion of a reaction

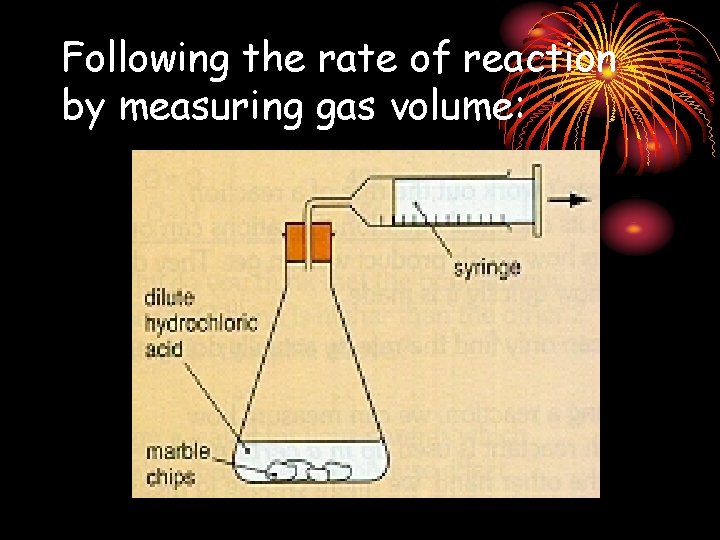
Following the rate of reaction by measuring gas volume:

Question : • 24 g of magnesium was added to an excess of dilute hydrochloric acid at r. t. p. Would there be a need to modify the set -up given earlier? Explain why. Mg + 2 HCl Mg. Cl 2 + H 2

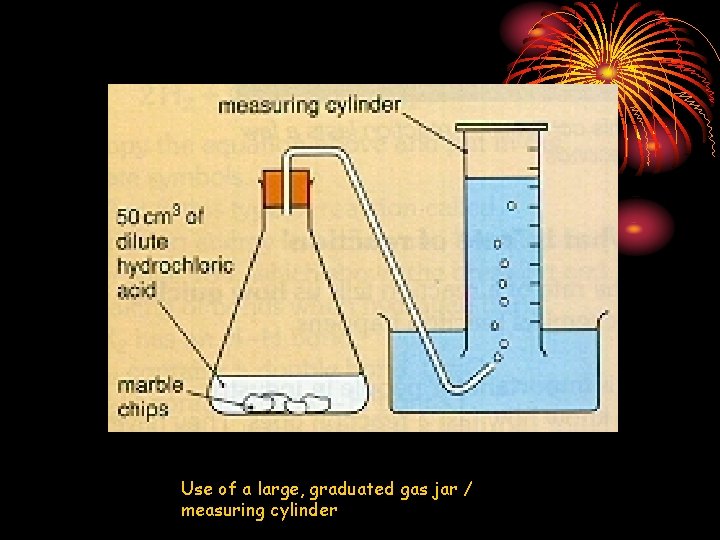
Use of a large, graduated gas jar / measuring cylinder

Following the rate of reaction by measuring mass of reactants over time:

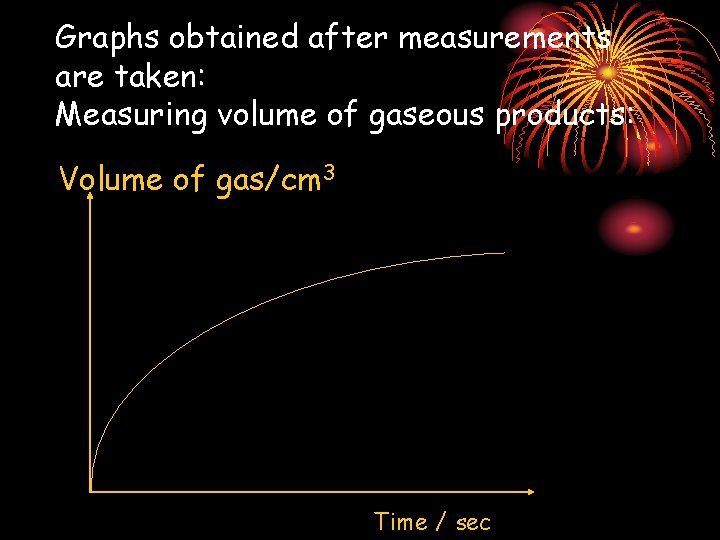
Graphs obtained after measurements are taken: Measuring volume of gaseous products: Volume of gas/cm 3 Time / sec

Measuring mass of reactants left over time: Mass of reactants / g Time / sec

Question: • Which reactions will give you the graph shown below ? Mass of reactants / g A. Acid – Metal B. Acid – Carbonate C. Acid- Alkali D. Precipitation Time

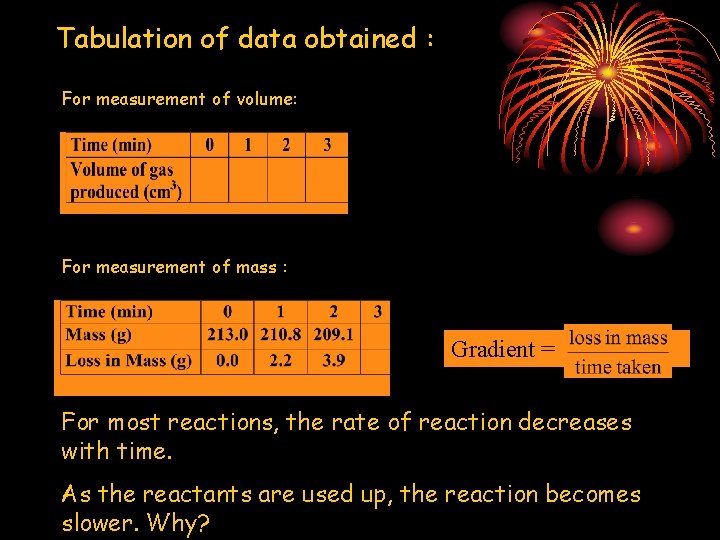
Tabulation of data obtained : For measurement of volume: For measurement of mass : Gradient = For most reactions, the rate of reaction decreases with time. As the reactants are used up, the reaction becomes slower. Why?

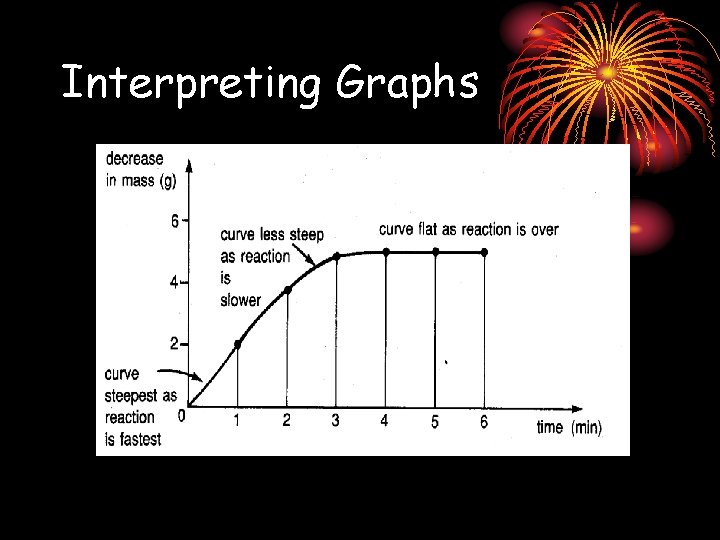
Interpreting Graphs

Interpreting Graphs • The rate of the reaction depends on the gradient of the graph. • Steeper gradient : Faster rate • In most reactions, the rate is fastest at the start of the reaction. Why? ?

• Because the concentration of the reactants is highest at the start, more collisions occur between reacting particles, enabling greater chances of effective collisions occurring between reacting particles. • Effective collisions occur when the reacting particles achieve the Activation energy required for reaction to occur.

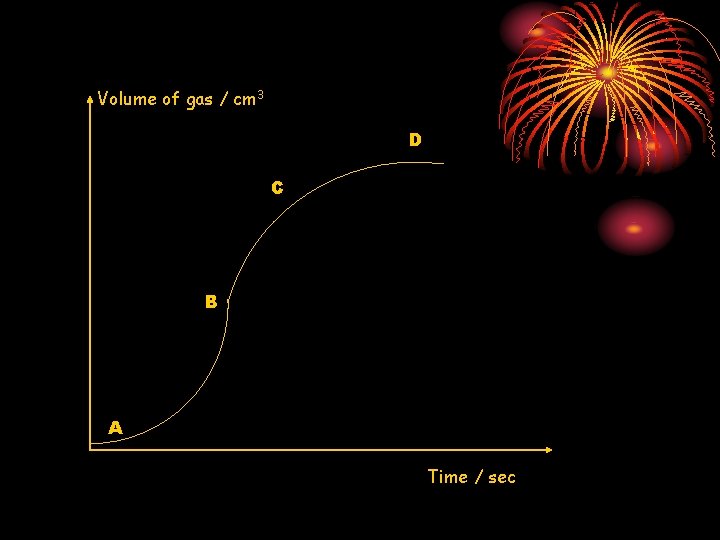
Volume of gas / cm 3 D C B A Time / sec

Analysis of graph • Rate is very slow at A, fastest at B, slows down at C and is zero at D. • This type of graph is possible if a metal coated with oxide layer is reacted with acid. The acid reacts with the oxide layer, removing it, after which, effervescence is observed as the exposed metal reacts with the acid.

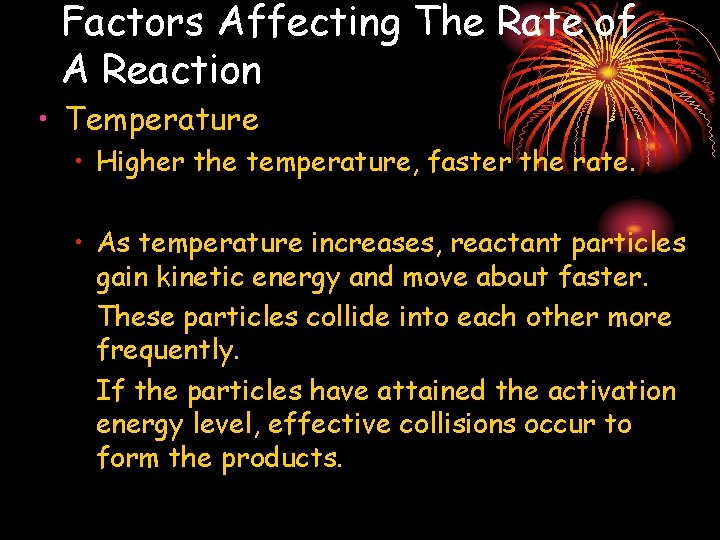
Factors Affecting The Rate of A Reaction • Temperature • Higher the temperature, faster the rate. • As temperature increases, reactant particles gain kinetic energy and move about faster. These particles collide into each other more frequently. If the particles have attained the activation energy level, effective collisions occur to form the products.

• Every 10 C increase in temperature doubles the rate of reaction. • Cooling a reaction mixture therefore reduces the rate of a reaction. • E. g. refrigeration of food

Question : 3 g of magnesium is reacted with 600 cm 3 of 0. 5 mol/dm 3 dilute hydrochloric acid at 30 C and 60 C. Which reactant is in excess? Which reaction would progress faster? How would these reactions be represented graphically?

• No. of moles of Mg No. of moles of HCl = 600/1000 × 0. 5 = 3 / 24 = 0. 125 mol = 0. 300 mol Mole ratio of Mg: HCl = 1 : 2 Therefore, 0. 125 moles of Mg reacts with 0. 250 moles of HCl. Since 0. 300 moles of HCl is greater than 0. 250 moles, HCl is in excess & Mg is the limiting reagent.

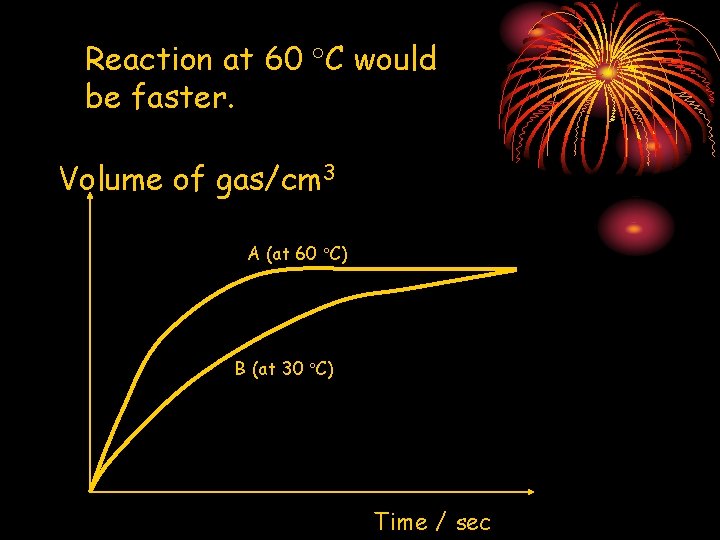
Reaction at 60 C would be faster. Volume of gas/cm 3 A (at 60 C) B (at 30 C) Time / sec

• Concentration of reactants (Solutions) • Higher the concentration , the faster the rate of the reaction. • When the reactant is concentrated, there are more particles present per unit volume of the solution. • Therefore, particles are closer together & there are greater chances of effective collisions of reactant particles occurring.

• Particle Size / Surface Area of Reactant particles • Reactants of smaller particle size have a larger total surface area exposed for a reaction to occur.

• The greater the surface area of the reactant particles (smaller particle size), the faster the rate of reaction. • Fine powders can react very fast i. e. explosively. • In flour mills, the finely powdered flour in the air can cause explosions & catch fire in the presence of a spark.

• Danger of explosions in coal mines • When methane is present (at 5% conc in air) with coal dust, the mixture is explosive. • The particle size of coal dust & the concentration of methane determine the chances of explosions occurring.

• Catalysts • A substance that alters the rate of a chemical reaction but remains chemically unchanged at the end of the reaction. • Its mass, density, chemical composition are unchanged at the end of the reaction.

• Only a small amount of the catalyst is required. Why? • Catalysts are specific in reaction i. e. a specific catalyst is used for a specific type of reaction.

• Some catalysts slow reactions down. These are negative catalysts or inhibitors. • If the surface area of the catalyst is increased (particle size is small), the catalyst is more effective in increasing the rate of the reaction.

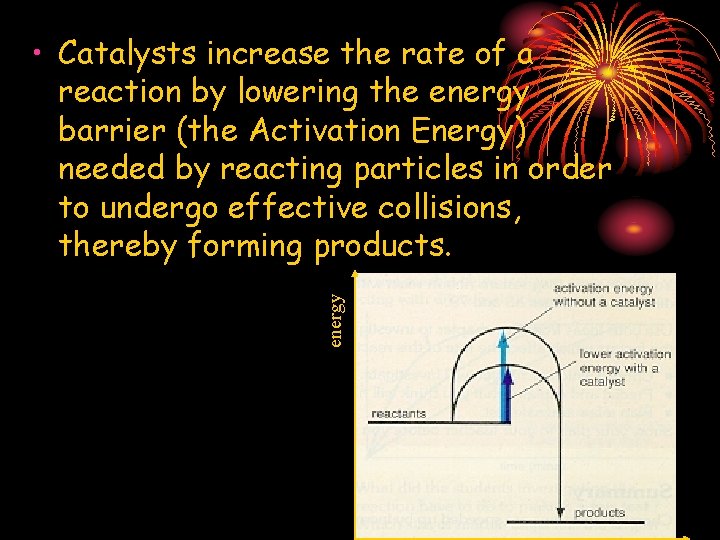
energy • Catalysts increase the rate of a reaction by lowering the energy barrier (the Activation Energy) needed by reacting particles in order to undergo effective collisions, thereby forming products.

Enzymes • Are biological catalysts. • They are protein molecules, which act on specific substrates. • They work best at optimum temperatures of 35 C to 40 C.

• If heated to higher temperatures, the enzymes are denatured. • At lower temperatures, the enzymes are inactive. • Enzymes work best within a narrow p. H range. E. g. pepsin in stomach requires an acidic medium to be effective.

• Pressure • Affects the reactions involving gases ONLY. • Increasing Pressure on a reacting mixture of gases increases the rate of the reaction.

As pressure is increased, the volume of the gaseous mixture decreases. The reacting particles are closer together & chances of effective collisions occurring increases. Therefore, rate of reaction will increase.

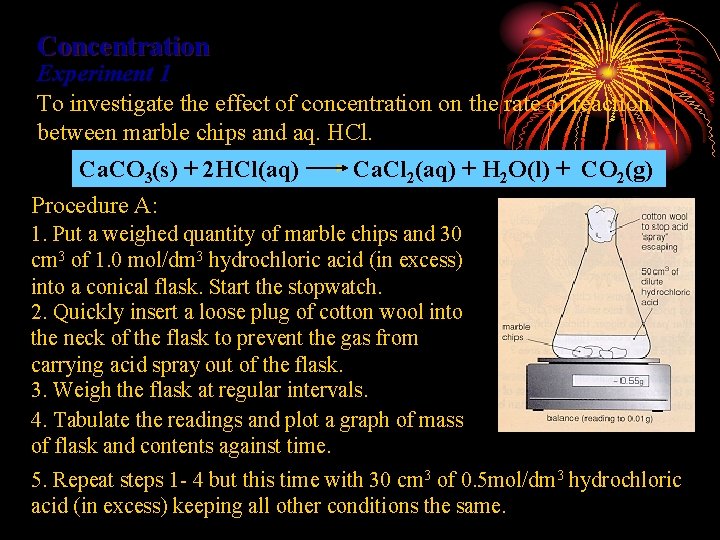
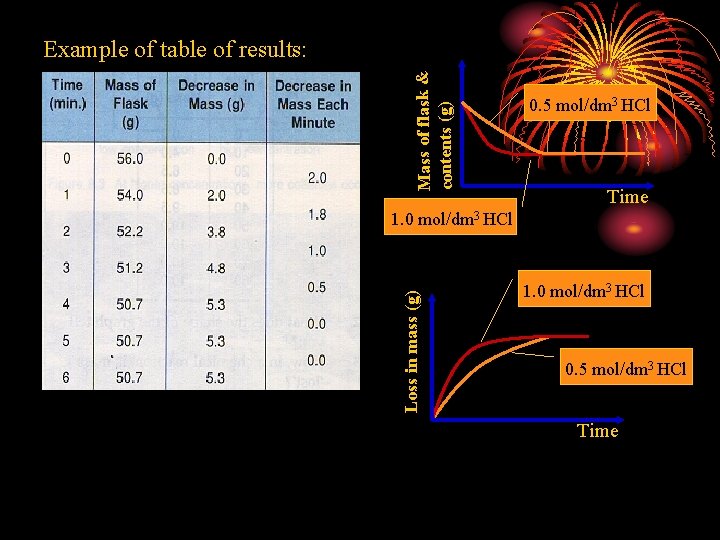
Concentration Experiment 1 To investigate the effect of concentration on the rate of reaction between marble chips and aq. HCl. Ca. CO 3(s) + 2 HCl(aq) Procedure A: Ca. Cl 2(aq) + H 2 O(l) + CO 2(g) 1. Put a weighed quantity of marble chips and 30 cm 3 of 1. 0 mol/dm 3 hydrochloric acid (in excess) into a conical flask. Start the stopwatch. 2. Quickly insert a loose plug of cotton wool into the neck of the flask to prevent the gas from carrying acid spray out of the flask. 3. Weigh the flask at regular intervals. 4. Tabulate the readings and plot a graph of mass of flask and contents against time. 5. Repeat steps 1 - 4 but this time with 30 cm 3 of 0. 5 mol/dm 3 hydrochloric acid (in excess) keeping all other conditions the same.

Mass of flask & contents (g) Example of table of results: 0. 5 mol/dm 3 HCl Time Loss in mass (g) 1. 0 mol/dm 3 HCl 0. 5 mol/dm 3 HCl Time

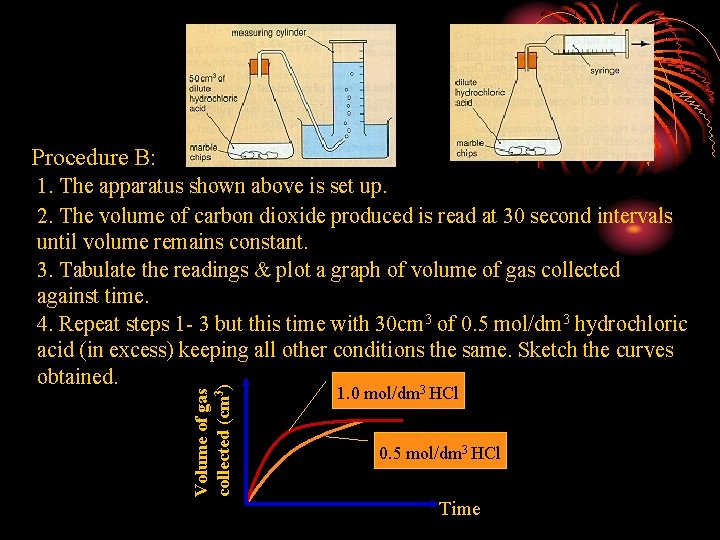
Procedure B: Volume of gas collected (cm 3) 1. The apparatus shown above is set up. 2. The volume of carbon dioxide produced is read at 30 second intervals until volume remains constant. 3. Tabulate the readings & plot a graph of volume of gas collected against time. 4. Repeat steps 1 - 3 but this time with 30 cm 3 of 0. 5 mol/dm 3 hydrochloric acid (in excess) keeping all other conditions the same. Sketch the curves obtained. 3 1. 0 mol/dm HCl 0. 5 mol/dm 3 HCl Time

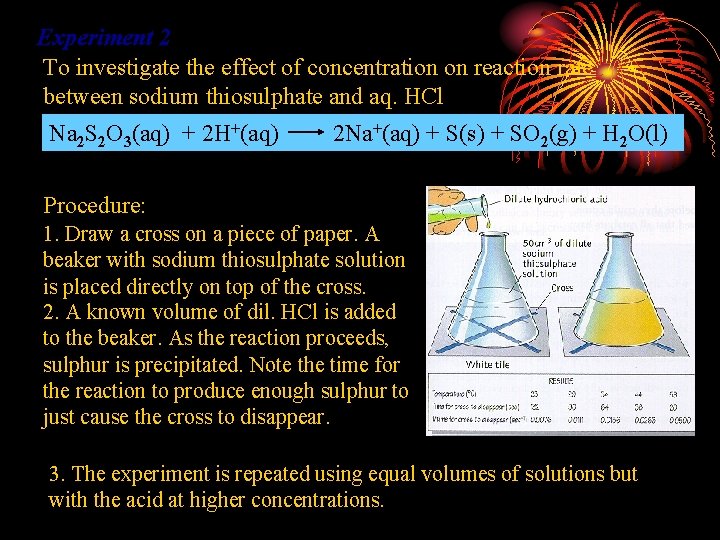
Experiment 2 To investigate the effect of concentration on reaction rate between sodium thiosulphate and aq. HCl Na 2 S 2 O 3(aq) + 2 H+(aq) 2 Na+(aq) + S(s) + SO 2(g) + H 2 O(l) Procedure: 1. Draw a cross on a piece of paper. A beaker with sodium thiosulphate solution is placed directly on top of the cross. 2. A known volume of dil. HCl is added to the beaker. As the reaction proceeds, sulphur is precipitated. Note the time for the reaction to produce enough sulphur to just cause the cross to disappear. 3. The experiment is repeated using equal volumes of solutions but with the acid at higher concentrations.

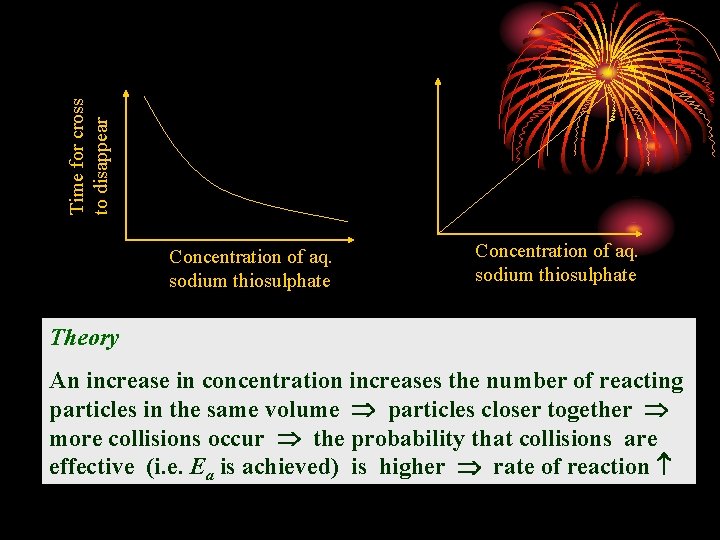
Time for cross to disappear Concentration of aq. sodium thiosulphate Theory An increase in concentration increases the number of reacting particles in the same volume particles closer together more collisions occur the probability that collisions are effective (i. e. Ea is achieved) is higher rate of reaction

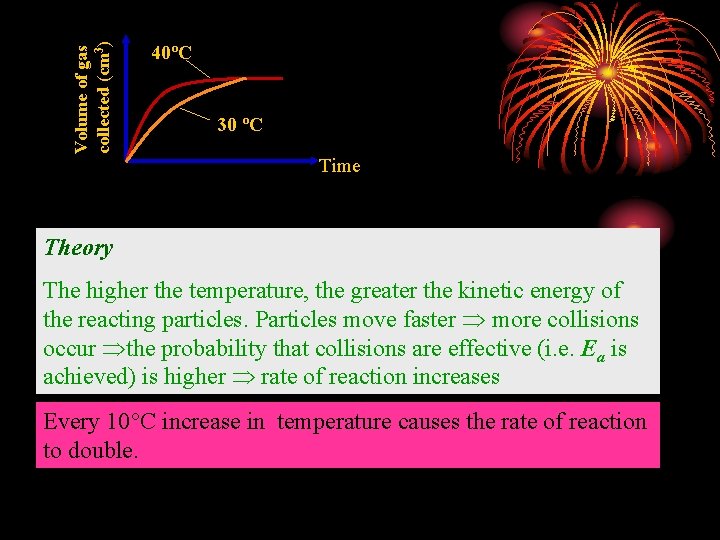
Temperature Experiment: To show the effect of temperature on the rate of reaction between magnesium and dil. hydrochloric acid. Procedure: 1. Pipette 20 cm 3 of dil. HCl of 0. 5 mol/dm 3 into a test-tube and place it in a water bath at room temperature. (30 ºC) 2. Quickly tip in 0. 1 g of Mg ribbon cut into small pieces and immediately replace the bung. 3. Read at 30 second intervals the volume of hydrogen collected in the syringe. Plot a graph of volume of hydrogen against time. 4. Using a fresh set of reactants each time repeat the experiment twice more with water baths of different temperatures. (40ºC)

Volume of gas collected (cm 3) 40ºC 30 ºC Time Theory The higher the temperature, the greater the kinetic energy of the reacting particles. Particles move faster more collisions occur the probability that collisions are effective (i. e. Ea is achieved) is higher rate of reaction increases Every 10°C increase in temperature causes the rate of reaction to double.

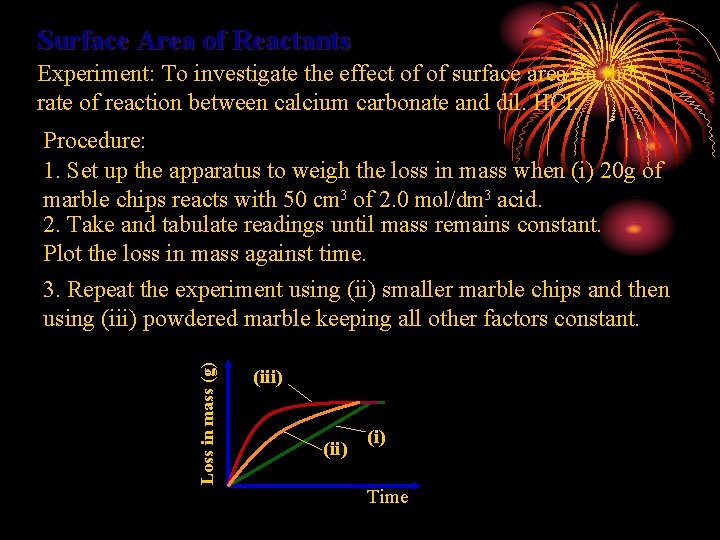
Surface Area of Reactants Experiment: To investigate the effect of of surface area on the rate of reaction between calcium carbonate and dil. HCl. Loss in mass (g) Procedure: 1. Set up the apparatus to weigh the loss in mass when (i) 20 g of marble chips reacts with 50 cm 3 of 2. 0 mol/dm 3 acid. 2. Take and tabulate readings until mass remains constant. Plot the loss in mass against time. 3. Repeat the experiment using (ii) smaller marble chips and then using (iii) powdered marble keeping all other factors constant. (iii) (i) Time

Theory The smaller the size of the solid reactant used the more surface area exposed more collisions occur the probability that collisions are effective (i. e. Ea is achieved) is higher rate of reaction increases The total surface are of the small particles is greater than that of the single large particle assuming same mass of substance. When a solid lump is cut into pieces, Its rate of reaction always increases. In the lump, most particles are locked up inside, In order to react, they have to collide! But powders have lots of particles exposed, If cut fine enough, they might even explode!

Catalysts A catalyst is substance that speeds up the rate of a reaction but remains chemically unchanged at the end of the reaction. Experiment: To investigate the effect of a catalyst on the rate of decomposition of hydrogen peroxide solution 2 H 2 O 2 (aq) 2 H 2 O(l) + O 2(g) Procedure 1. Put a little manganese dioxide powder (black) in the conical flask, then pour in the H 2 O 2 solution. 2. Note the volume of oxygen collected at 1 minute intervals, tabulate results and plot a graph of volume of oxygen against time 3. Repeat the experiment using the same volume of H 2 O 2 but in the absence of the catalyst.

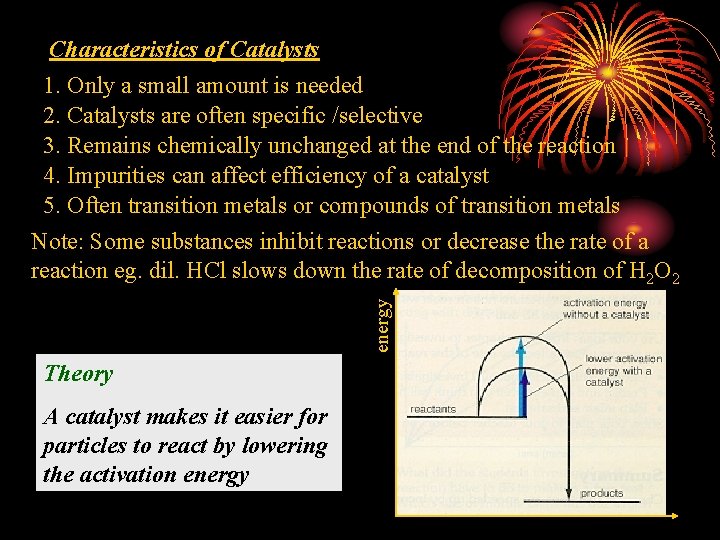
energy Characteristics of Catalysts 1. Only a small amount is needed 2. Catalysts are often specific /selective 3. Remains chemically unchanged at the end of the reaction 4. Impurities can affect efficiency of a catalyst 5. Often transition metals or compounds of transition metals Note: Some substances inhibit reactions or decrease the rate of a reaction eg. dil. HCl slows down the rate of decomposition of H 2 O 2 Theory A catalyst makes it easier for particles to react by lowering the activation energy

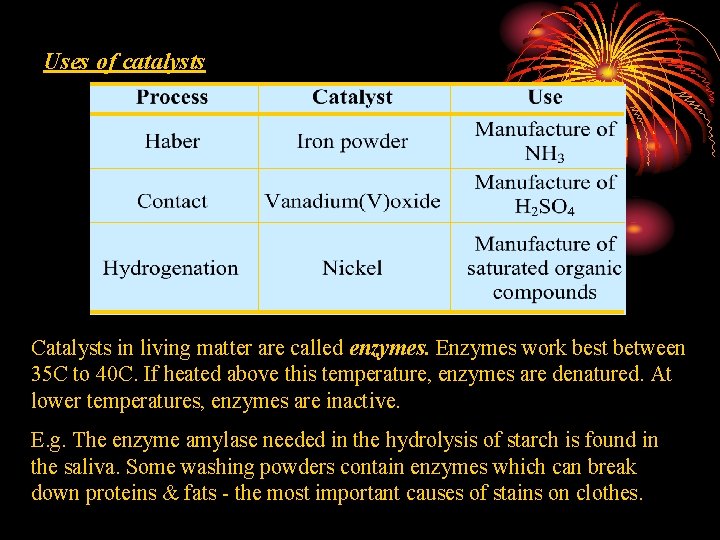
Uses of catalysts Catalysts in living matter are called enzymes. Enzymes work best between 35 C to 40 C. If heated above this temperature, enzymes are denatured. At lower temperatures, enzymes are inactive. E. g. The enzyme amylase needed in the hydrolysis of starch is found in the saliva. Some washing powders contain enzymes which can break down proteins & fats - the most important causes of stains on clothes.

Light Experiment: To investigate the effect of light on silver halides. Procedure: 1. Pour dilute solutions of KCl, KBr and KI into two test-tubes each. 2. Add Ag. NO 3, solution to each test-tube. 3. Silver halides will be precipitated. Immediately place one testtube of each silver halide into a darkened cupboard and the other near a window. 4. Observe what happens after 15 minutes Results: The test-tubes in the cupboard remain the same while those near the window have darkened due to the reduction of silver halides to silver metal.

Type of Bond The rate of reaction involving breaking and forming of ionic bonds is faster than that involving covalent bonds. Formation of new ionic compounds in precipitation reactions is almost instantaneous while formation of new covalent organic compounds requires high temperatures, catalyst, etc.

Applications • Use of catalyst in industry • Catalysts increase the rate of a reaction by lowering the Activation energy of the reaction. • Catalysts are specific in reaction − Iron in Haber Process (Production of Ammonia) − Platinum in Catalytic Converters (Removal of Oxides of Nitrogen & Carbon Monoxide) − Vanadium (V) oxide in the Contact Process (Conversion of Sulphur dioxide to Sulphur trioxide)

Applications • Use of catalyst in industry • Small pellets of catalyst are used rather than lumps, greater surface area of catalyst; faster rate of reaction • Platinum / Rhodium / Palladium is coated on ceramic, increases surface area of catalyst, faster rate of reaction in catalytic converters • Cooking of food • Higher temperature, faster cooking of food • Smaller pieces of food, smaller particle size, greater surface area, faster rate of cooking of food • High pressure, higher temperature, faster rate of cooking food

Applications • Storage of tapes, cassettes, & books in libraries • Lower temperature, slower rate of spoilage of materials by fungal attack • Air-conditioning lowers moisture content of air; lowers chances of growth of fungus

Applications • Fine Dust in the Air • Explosions can occur if the dust combines with oxygen when a spark is applied • Small particle size of dust, greater surface area, faster rate of combustion • Examples : Coal dust in coal mines Flour dust in flour mills Curry powder dust in curry powder mills

Applications • Explosions in Coal Mines • Explosions can occur if the mines are not wellventilated • There is accumulation of combustible gases (methane) in coal mines • High concentrations of methane increase the risk of explosions.

Applications • Oxygen tents / Petrol Stations • Threat of fire / explosions due to high concentration of oxygen gas present in oxygen tents • Warnings not to smoke / light a naked flame at petrol stations due to risk of explosion caused by high concentrations of petrol vapour present

Applications • In Antibiotics • Fungi produce enzymes which kill bacteria. These enzymes are called antibiotics • In Fermentation • Yeast cells produce enzymes (biological catalysts) that convert sugars to ethanol & carbon dioxide