RATES OF REACTIONS Lycopodium dust explosion https youtu










- Slides: 38

RATES OF REACTIONS

Lycopodium dust explosion: https: //youtu. be/TAd. El. O 1 FCSM

There are three types of catalysis: 1. Homogeneous catalysis 2. Heterogeneous catalysis 3. Autocatalysis

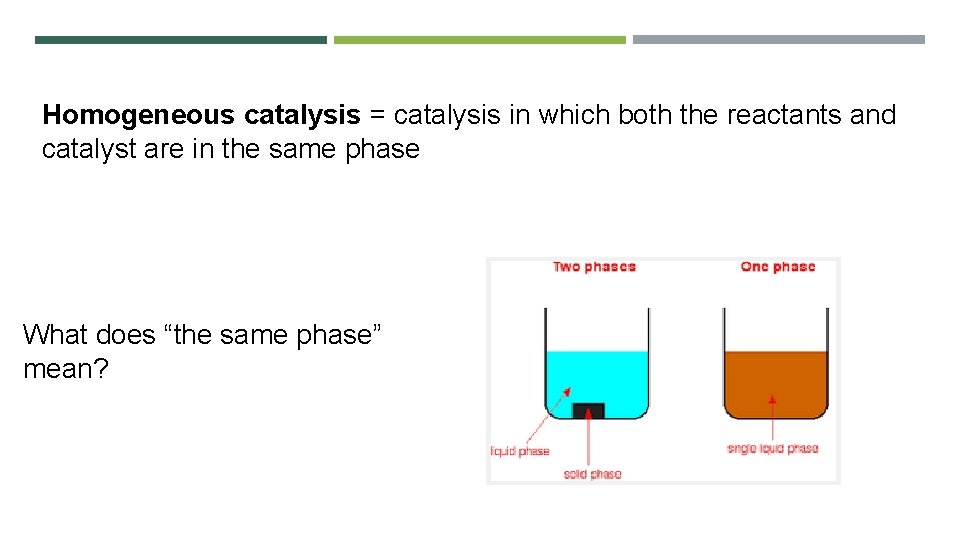
Homogeneous catalysis = catalysis in which both the reactants and catalyst are in the same phase What does “the same phase” mean?

Are these liquids in the same phase? Ans: No. There is a boundary between the two liquids

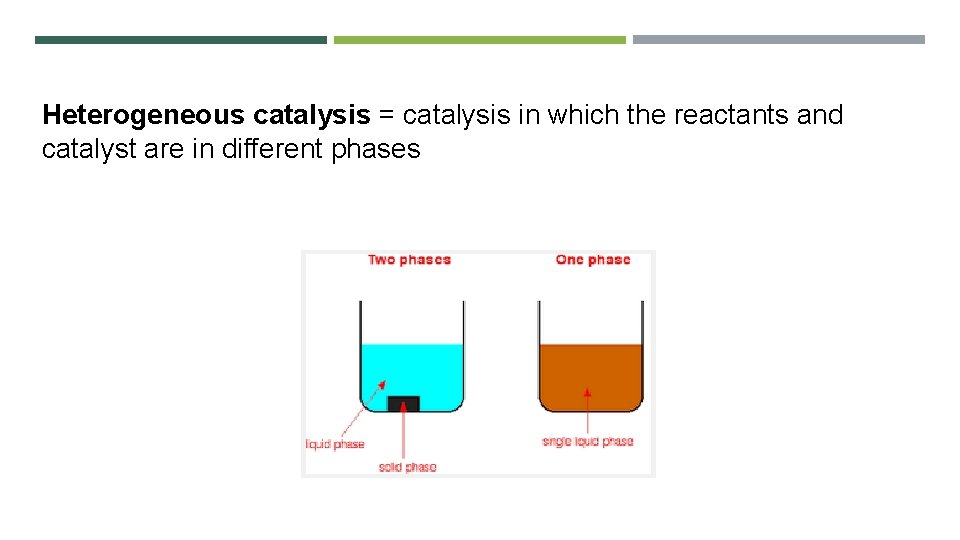
Heterogeneous catalysis = catalysis in which the reactants and catalyst are in different phases

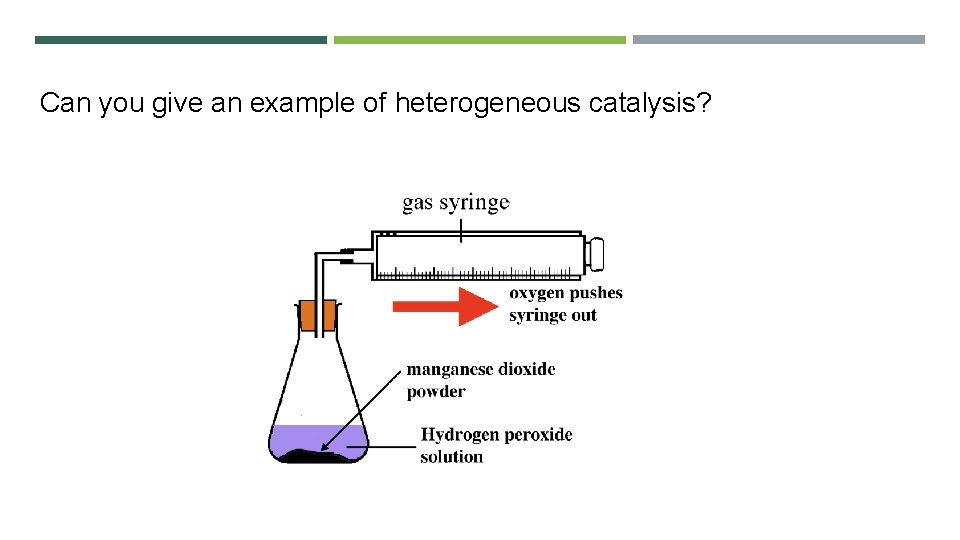
Can you give an example of heterogeneous catalysis?

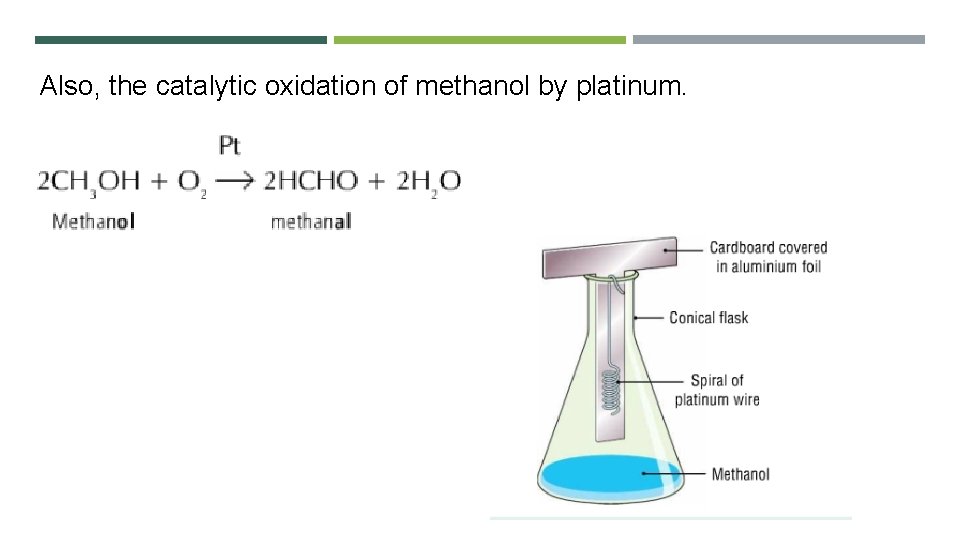
Also, the catalytic oxidation of methanol by platinum.

The catalytic oxidation of methanol by platinum. https: //youtu. be/FSd. BB 1 v. BDKY

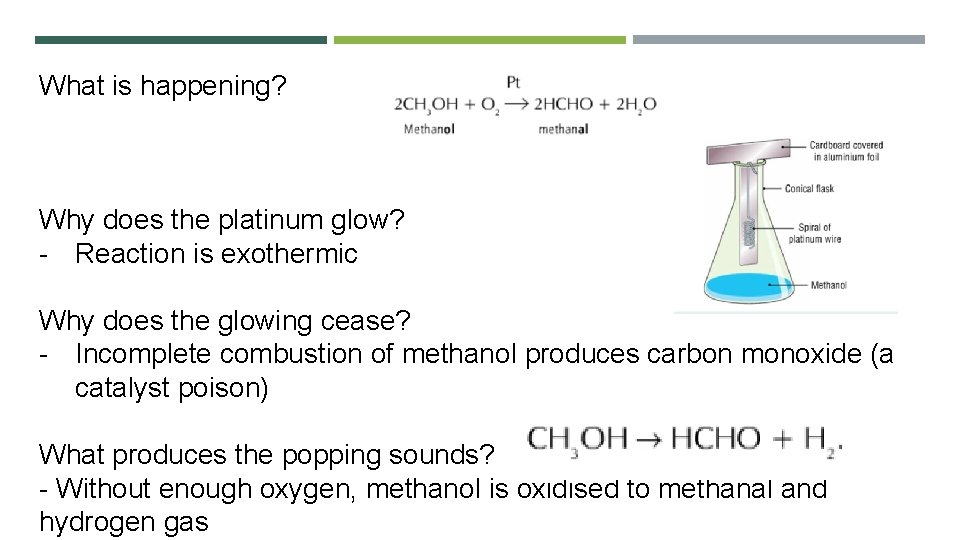
What is happening? Why does the platinum glow? - Reaction is exothermic Why does the glowing cease? - Incomplete combustion of methanol produces carbon monoxide (a catalyst poison) What produces the popping sounds? - Without enough oxygen, methanol is oxidised to methanal and hydrogen gas

Autocatalysis = catalysis in which one of the products of the reaction acts as a catalyst for the reaction Where did you see this before?

Mechanisms of Catalysis 1. Intermediate formation theory 2. Surface adsorption theory

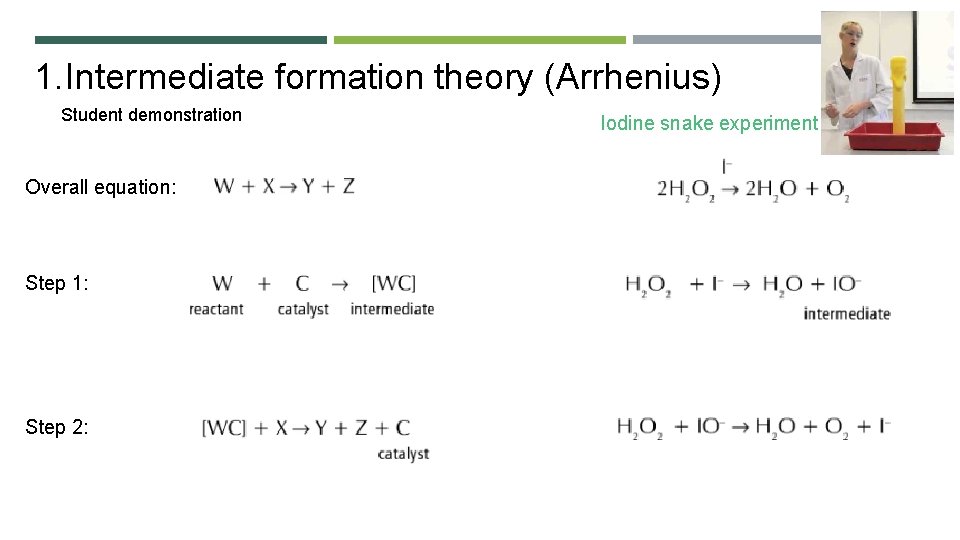
1. Intermediate formation theory (Arrhenius) Student demonstration Overall equation: Step 1: Step 2: Iodine snake experiment

Evidence for the Intermediate Formation Theory of Catalysis: https: //youtu. be/V 3 l. NGp-7 kp 4 Reaction of potassium sodium tartrate and hydrogen peroxide, catalysed by Co 2+ ions

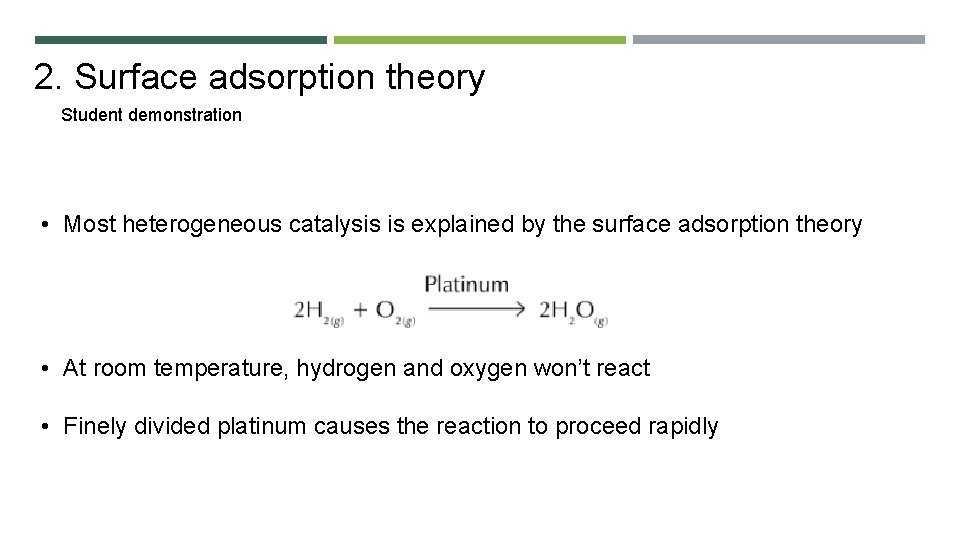
2. Surface adsorption theory Student demonstration • Most heterogeneous catalysis is explained by the surface adsorption theory • At room temperature, hydrogen and oxygen won’t react • Finely divided platinum causes the reaction to proceed rapidly

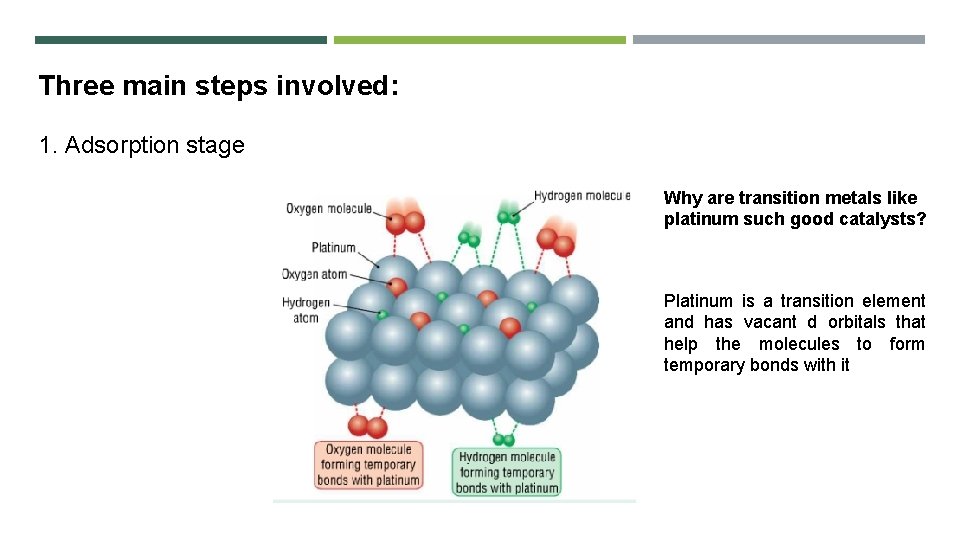
Three main steps involved: 1. Adsorption stage Why are transition metals like platinum such good catalysts? Platinum is a transition element and has vacant d orbitals that help the molecules to form temporary bonds with it

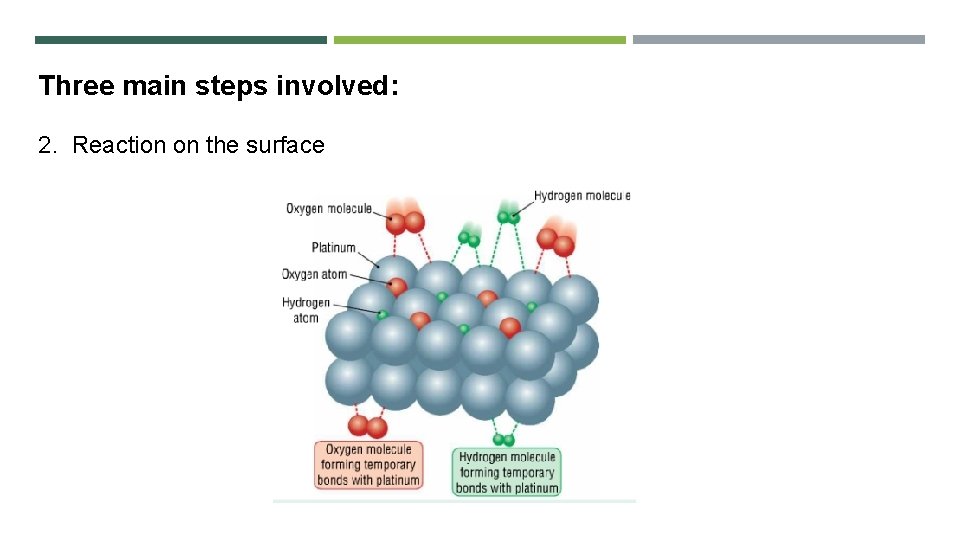
Three main steps involved: 2. Reaction on the surface

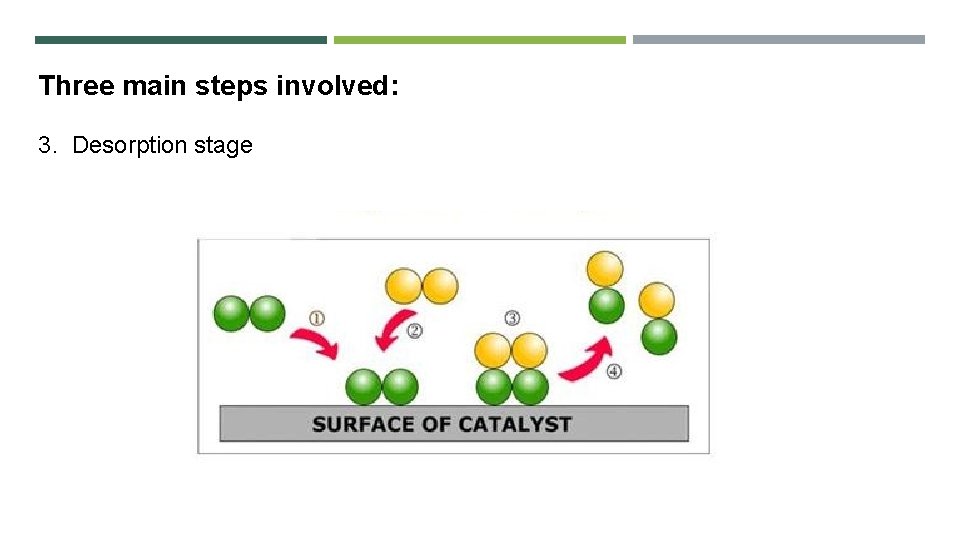
Three main steps involved: 3. Desorption stage

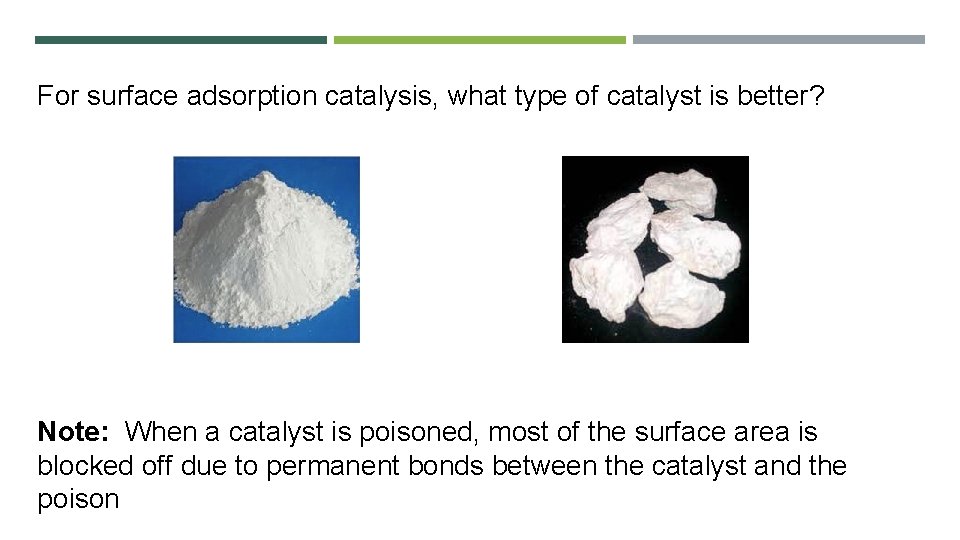
For surface adsorption catalysis, what type of catalyst is better? Note: When a catalyst is poisoned, most of the surface area is blocked off due to permanent bonds between the catalyst and the poison

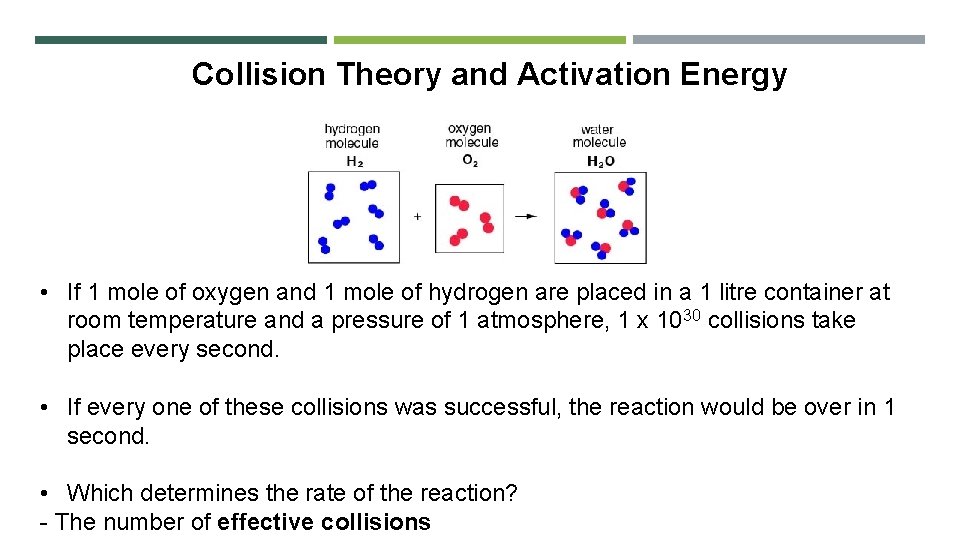
Collision Theory and Activation Energy Collison theory says that: 1. For a reaction to occur, the reacting particles must collide with each other 2. A collision only results in the formation of products if a certain minimum energy is exceeded in the collision

Collision Theory and Activation Energy • If 1 mole of oxygen and 1 mole of hydrogen are placed in a 1 litre container at room temperature and a pressure of 1 atmosphere, 1 x 1030 collisions take place every second. • If every one of these collisions was successful, the reaction would be over in 1 second. • Which determines the rate of the reaction? - The number of effective collisions

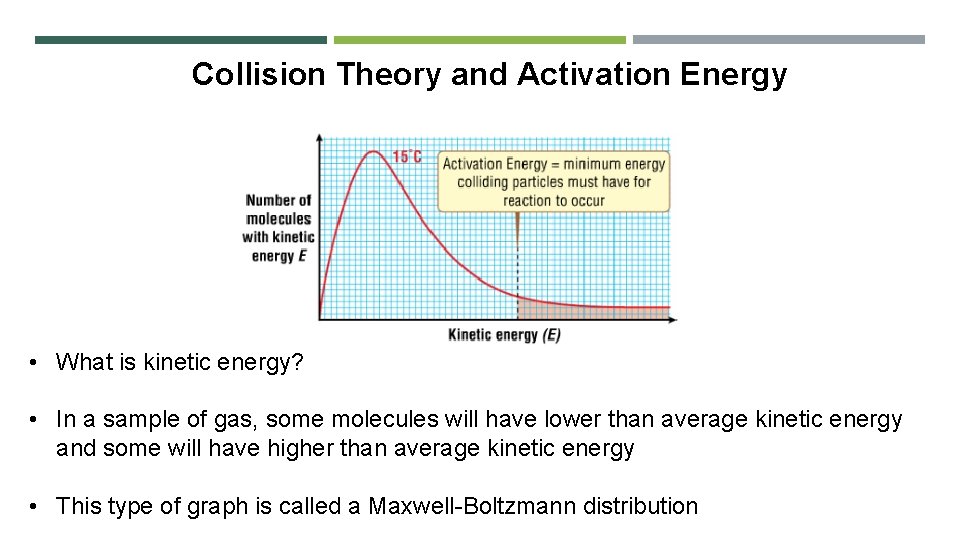
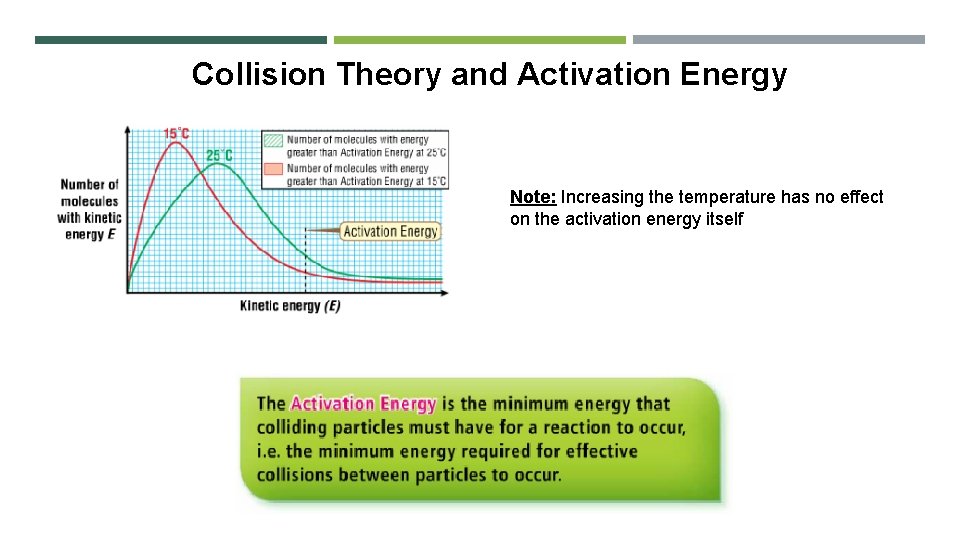
Collision Theory and Activation Energy • What is kinetic energy? • In a sample of gas, some molecules will have lower than average kinetic energy and some will have higher than average kinetic energy • This type of graph is called a Maxwell-Boltzmann distribution

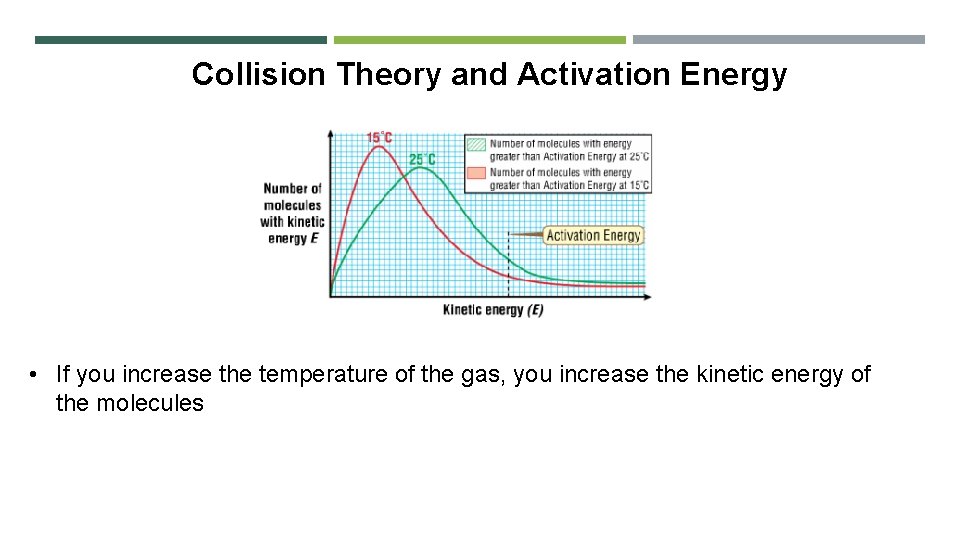
Collision Theory and Activation Energy • If you increase the temperature of the gas, you increase the kinetic energy of the molecules

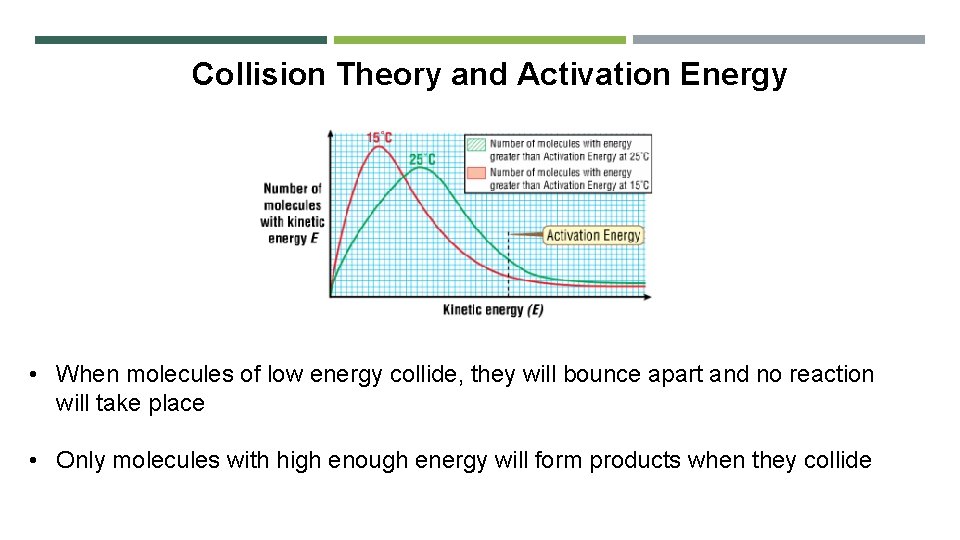
Collision Theory and Activation Energy • When molecules of low energy collide, they will bounce apart and no reaction will take place • Only molecules with high enough energy will form products when they collide

Collision Theory and Activation Energy Note: Increasing the temperature has no effect on the activation energy itself

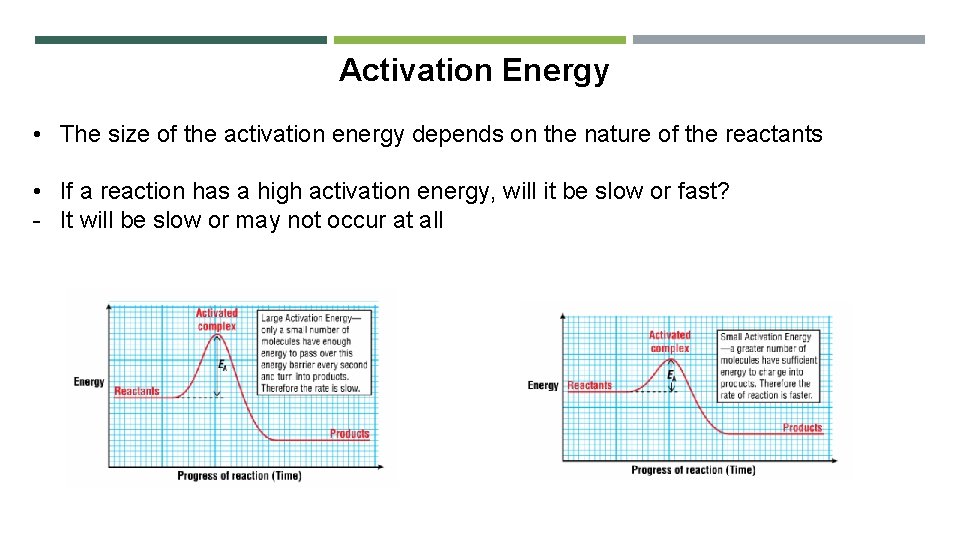
Activation Energy • The size of the activation energy depends on the nature of the reactants • If a reaction has a high activation energy, will it be slow or fast? - It will be slow or may not occur at all

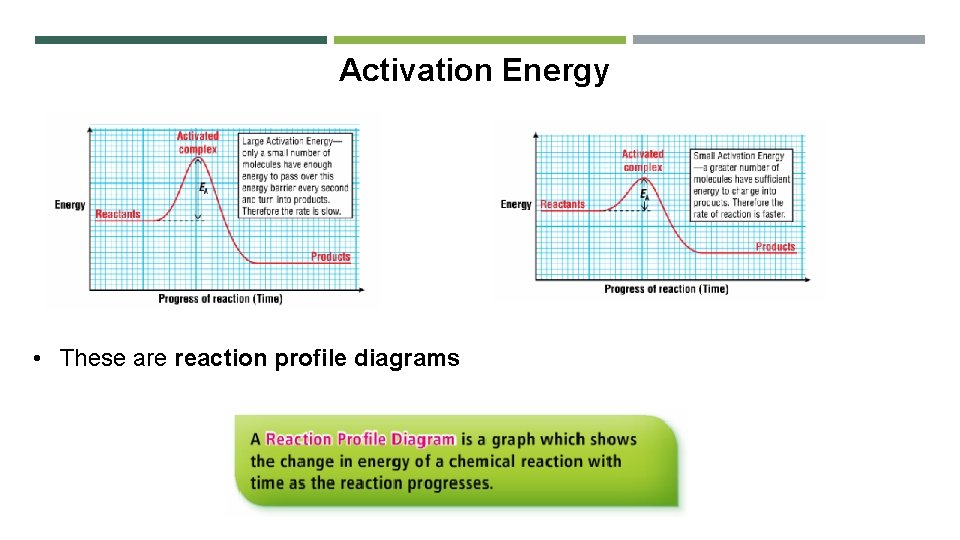
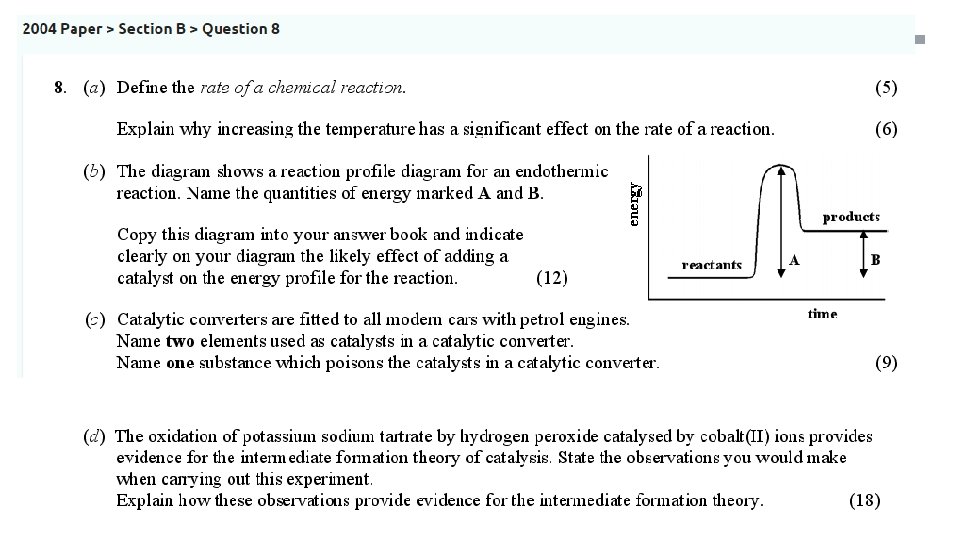
Activation Energy • These are reaction profile diagrams

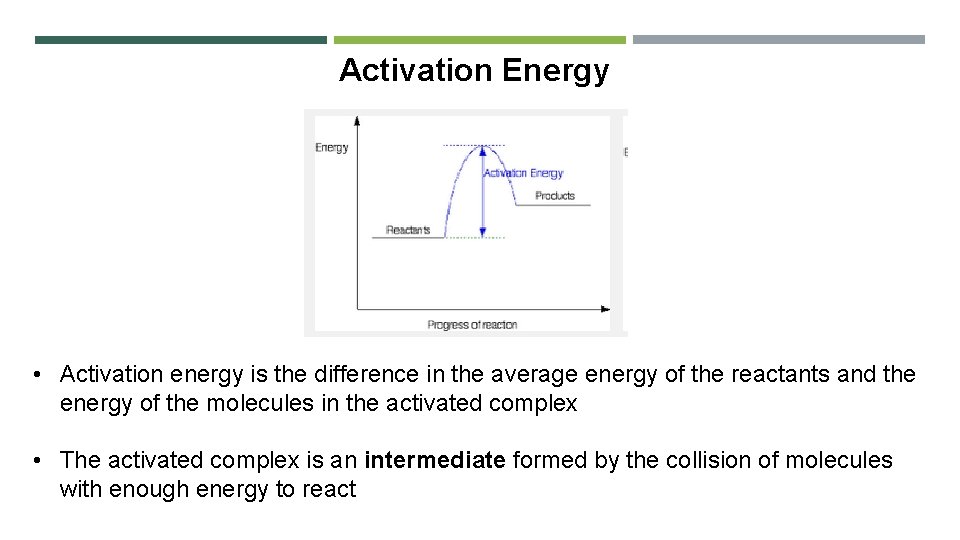
Activation Energy • Activation energy is the difference in the average energy of the reactants and the energy of the molecules in the activated complex • The activated complex is an intermediate formed by the collision of molecules with enough energy to react

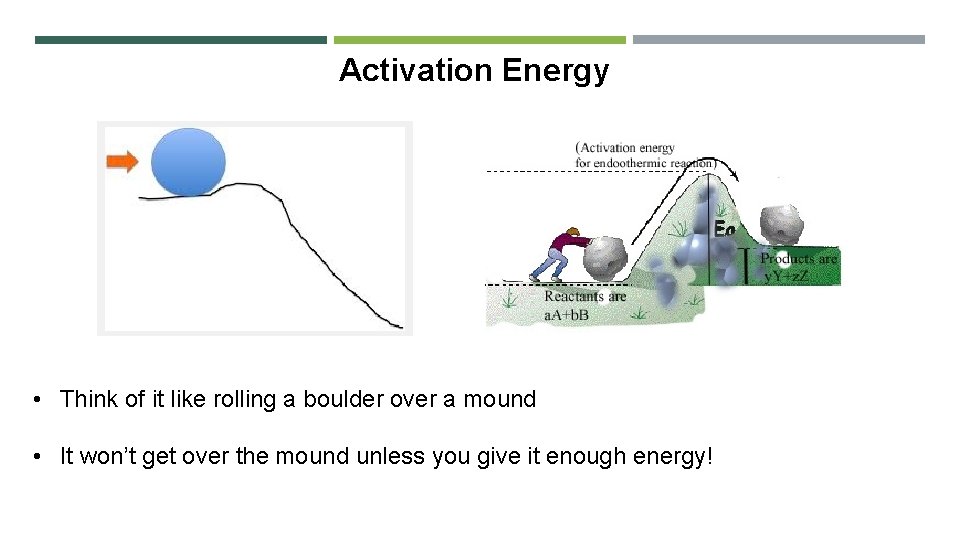
Activation Energy • Think of it like rolling a boulder over a mound • It won’t get over the mound unless you give it enough energy!

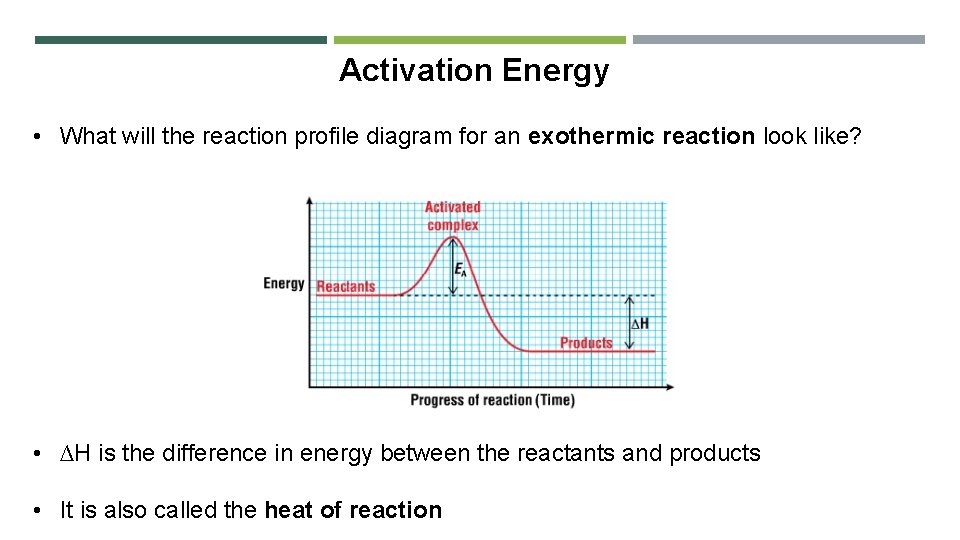
Activation Energy • What will the reaction profile diagram for an exothermic reaction look like? • ∆H is the difference in energy between the reactants and products • It is also called the heat of reaction

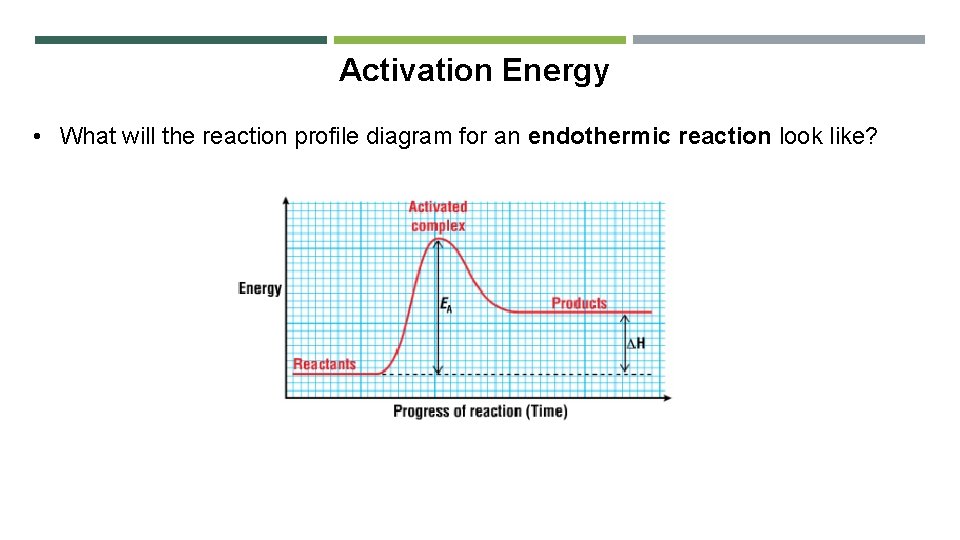
Activation Energy • What will the reaction profile diagram for an endothermic reaction look like?

How does collision theory explain other factors that affect the rate of a reaction? • Nature of reactants Covalent compounds require bond breaking, which requires more energy than reactions in which ions are simply coming together • Particle size Smaller particles have a greater surface area and so more collisions take place If the number of collisions increases, the number of effective collisions will also

How does collision theory explain other factors that affect the rate of a reaction? • Concentration If the number of particles increases, the number of collisions increases • Catalyst A catalyst provides an alternative reaction pathway with lower activation energy

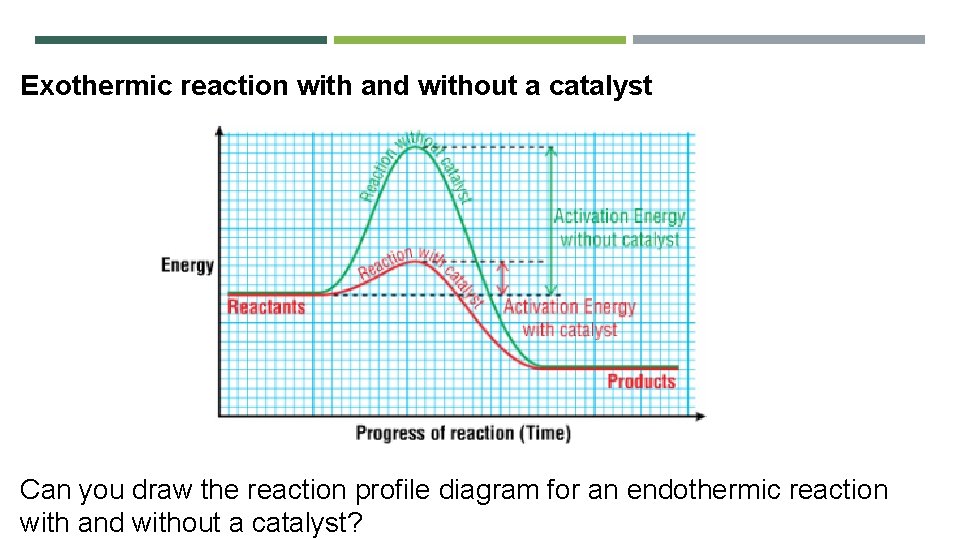
Exothermic reaction with and without a catalyst Can you draw the reaction profile diagram for an endothermic reaction with and without a catalyst?

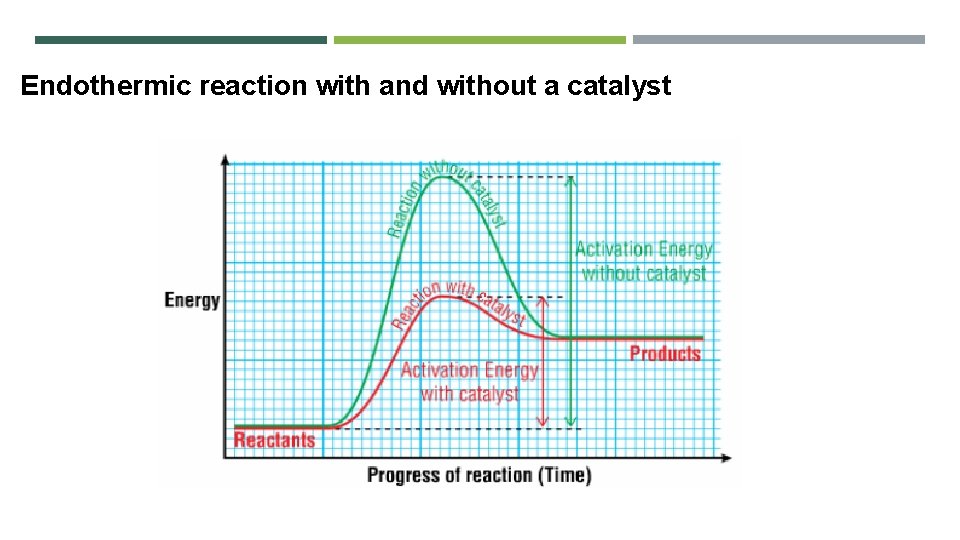
Endothermic reaction with and without a catalyst


