Rates of Reactions and Enzymes Rates of Reaction



- Slides: 10

Rates of Reactions and Enzymes

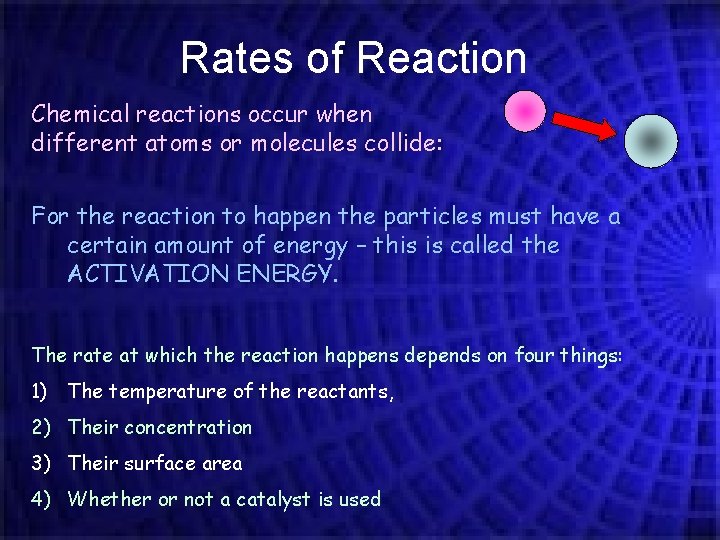
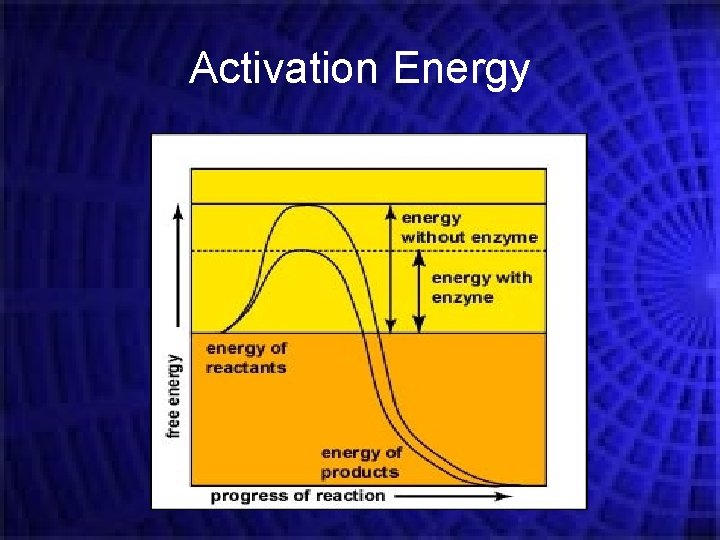
Rates of Reaction Chemical reactions occur when different atoms or molecules collide: For the reaction to happen the particles must have a certain amount of energy – this is called the ACTIVATION ENERGY. The rate at which the reaction happens depends on four things: 1) The temperature of the reactants, 2) Their concentration 3) Their surface area 4) Whether or not a catalyst is used

Energy in reactions • In all chemical reactions, energy is either released or absorbed. • When bonds are broken, energy is released. • When bonds are formed, energy is stored. • Living things require a constant source of energy input to keep the chemical reactions going. (plants use the sun, etc. )

Activation Energy

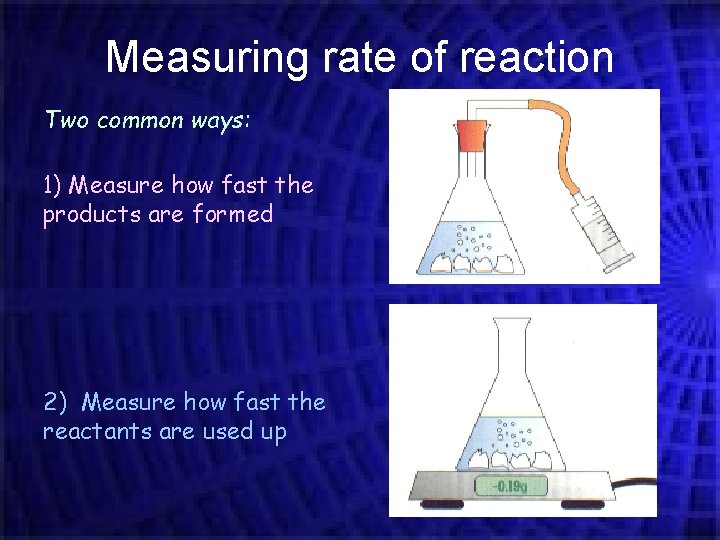
Measuring rate of reaction Two common ways: 1) Measure how fast the products are formed 2) Measure how fast the reactants are used up

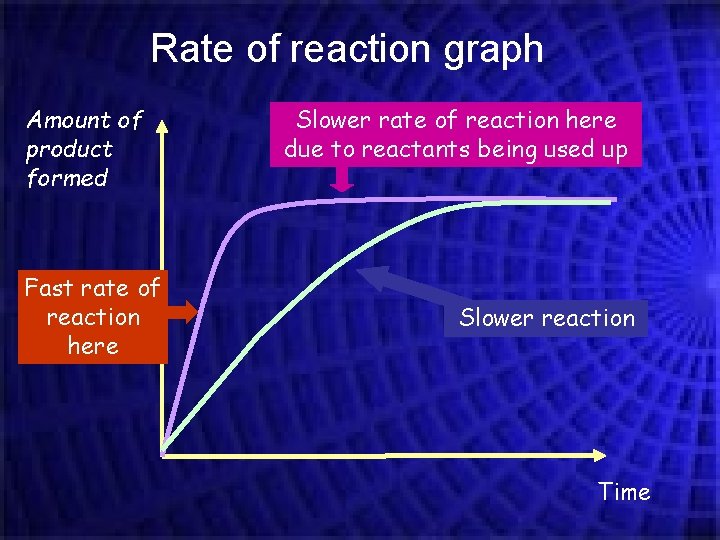
Rate of reaction graph Amount of product formed Fast rate of reaction here Slower rate of reaction here due to reactants being used up Slower reaction Time

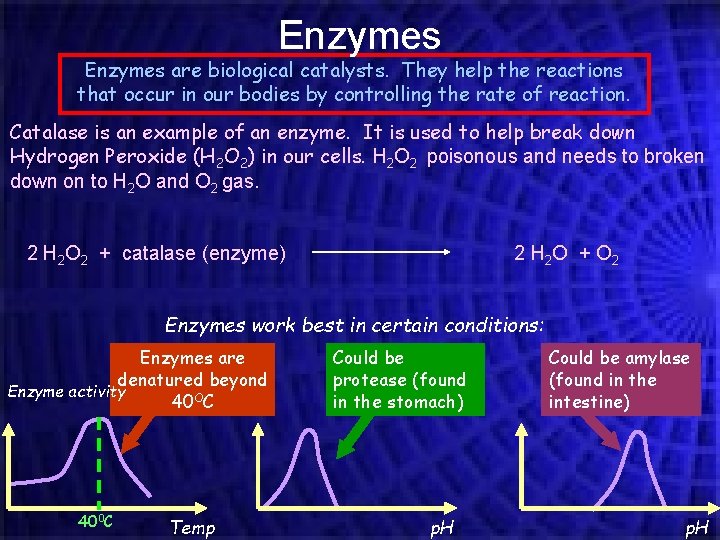
Enzymes are biological catalysts. They help the reactions that occur in our bodies by controlling the rate of reaction. Catalase is an example of an enzyme. It is used to help break down Hydrogen Peroxide (H 2 O 2) in our cells. H 2 O 2 poisonous and needs to broken down on to H 2 O and O 2 gas. 2 H 2 O 2 + catalase (enzyme) 2 H 2 O + O 2 Enzymes work best in certain conditions: Enzymes are denatured beyond Enzyme activity 40 OC 400 C Temp Could be protease (found in the stomach) p. H Could be amylase (found in the intestine) p. H

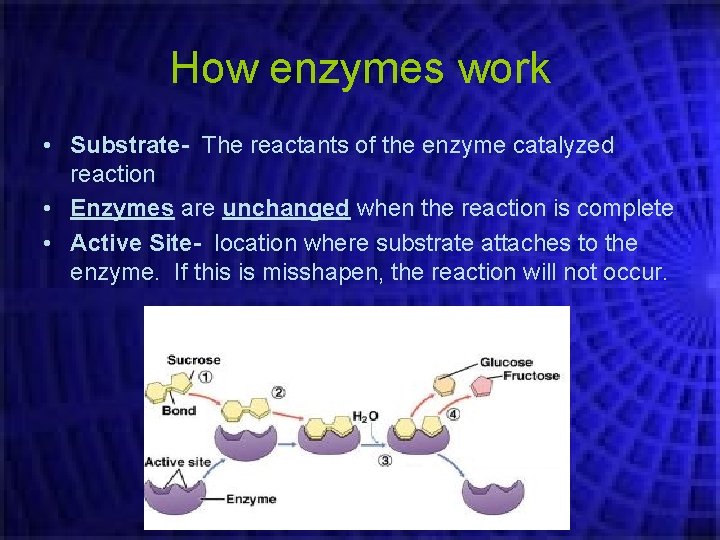
How enzymes work • Substrate- The reactants of the enzyme catalyzed reaction • Enzymes are unchanged when the reaction is complete • Active Site- location where substrate attaches to the enzyme. If this is misshapen, the reaction will not occur.

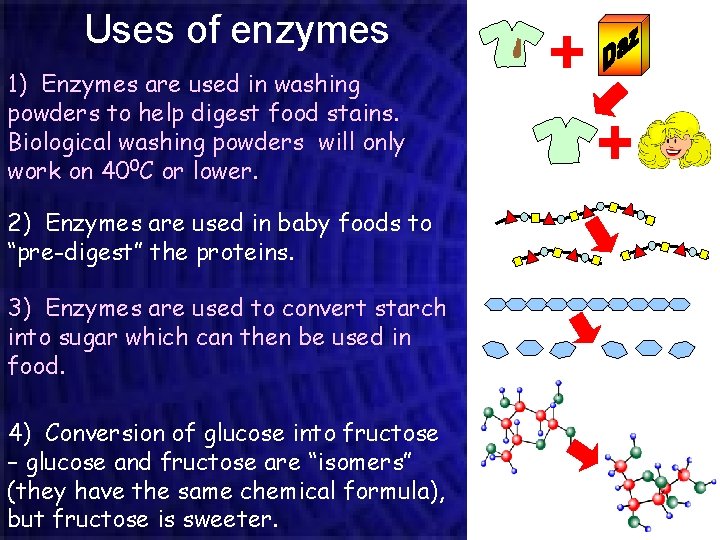
Uses of enzymes 1) Enzymes are used in washing powders to help digest food stains. Biological washing powders will only work on 400 C or lower. 2) Enzymes are used in baby foods to “pre-digest” the proteins. 3) Enzymes are used to convert starch into sugar which can then be used in food. 4) Conversion of glucose into fructose – glucose and fructose are “isomers” (they have the same chemical formula), but fructose is sweeter.

This powerpoint was kindly donated to www. worldofteaching. com http: //www. worldofteaching. com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching.
 Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Biology-roots.com
Biology-roots.com Enzyme catalyzed reaction
Enzyme catalyzed reaction Is a ratio a rate
Is a ratio a rate Ratios guided notes
Ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Balancing redox reactions
Balancing redox reactions