Rates of reaction project Starter The chemical industry
- Slides: 8

Rates of reaction project Starter: The chemical industry makes plastics, acids, fertilisers, pesticides, pharmaceuticals and many others. 1. Why might companies want to speed up the reactions that make these products? 2. Why might companies want to slow down the reaction to make these products? 3. How might a chemist go about measuring the speed of a reaction?

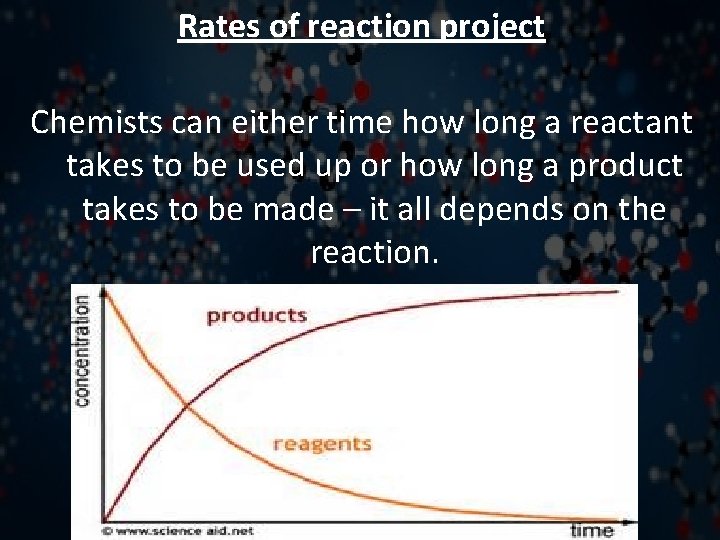
Rates of reaction project Chemists can either time how long a reactant takes to be used up or how long a product takes to be made – it all depends on the reaction.

Rates of reaction project Our reaction is going to be between magnesium and hydrochloric acid: magnesium + hydrochloric acid → magnesium chloride + hydrogen Mg(s) + HCl(aq) → Mg. Cl 2(aq) + H 2(g) solid colourless solution gas How could we measure the speed of the reaction?

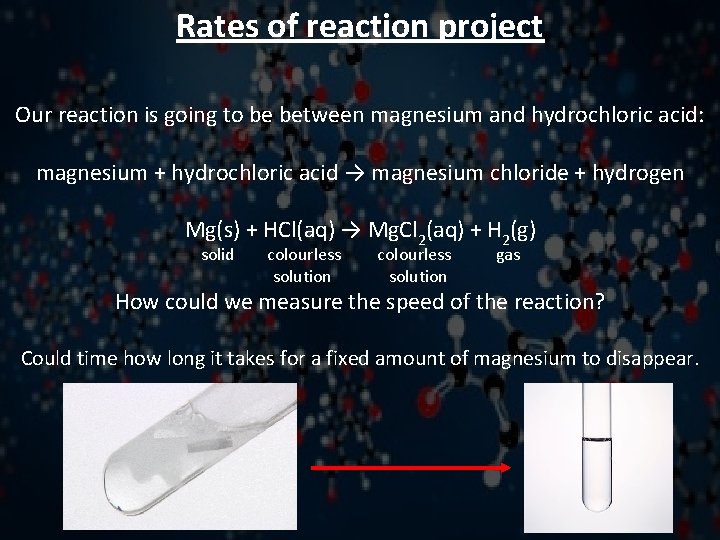
Rates of reaction project Our reaction is going to be between magnesium and hydrochloric acid: magnesium + hydrochloric acid → magnesium chloride + hydrogen Mg(s) + HCl(aq) → Mg. Cl 2(aq) + H 2(g) solid colourless solution gas How could we measure the speed of the reaction? Could time how long it takes for a fixed amount of magnesium to disappear.

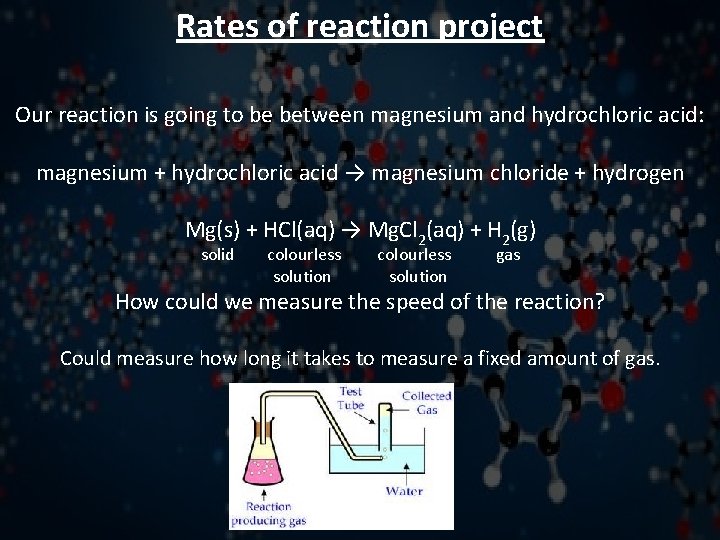
Rates of reaction project Our reaction is going to be between magnesium and hydrochloric acid: magnesium + hydrochloric acid → magnesium chloride + hydrogen Mg(s) + HCl(aq) → Mg. Cl 2(aq) + H 2(g) solid colourless solution gas How could we measure the speed of the reaction? Could measure how long it takes to measure a fixed amount of gas.

Rates of reaction project We are going to see if changing the concentration of the hydrochloric acid changes the speed of the reaction. magnesium + hydrochloric acid → magnesium chloride + hydrogen Mg(s) + HCl(aq) → Mg. Cl 2(aq) + H 2(g) solid colourless solution gas

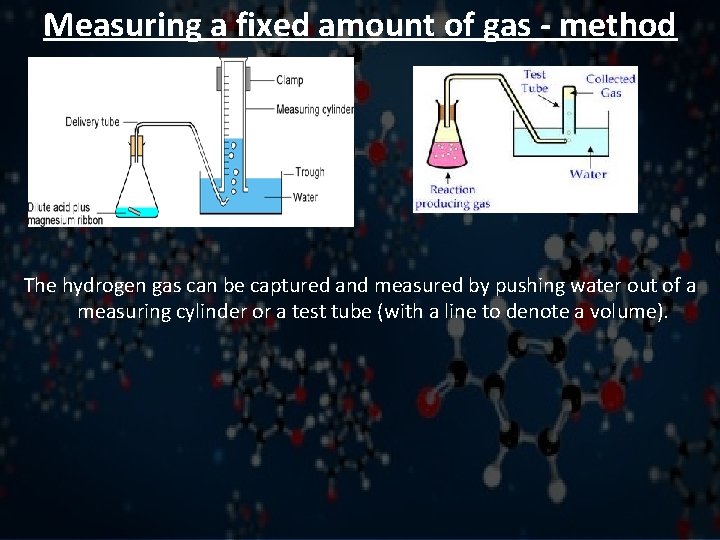
Measuring a fixed amount of gas - method The hydrogen gas can be captured and measured by pushing water out of a measuring cylinder or a test tube (with a line to denote a volume).

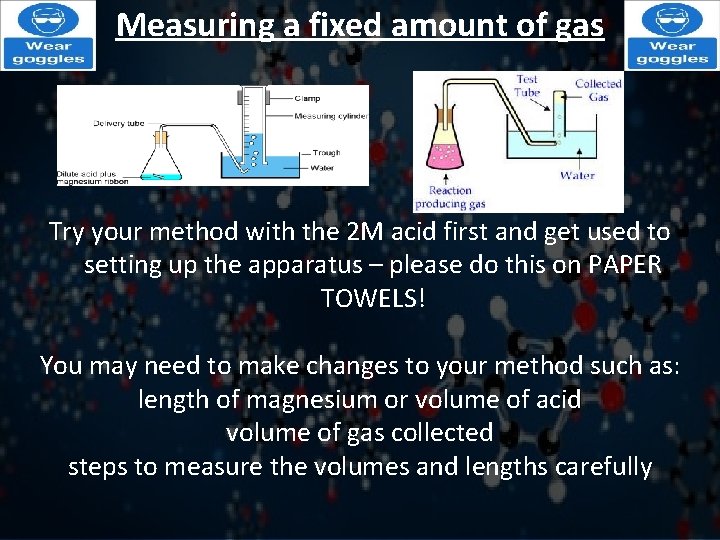
Measuring a fixed amount of gas Try your method with the 2 M acid first and get used to setting up the apparatus – please do this on PAPER TOWELS! You may need to make changes to your method such as: length of magnesium or volume of acid volume of gas collected steps to measure the volumes and lengths carefully