Rates of Reaction How do we calculate Rate








- Slides: 25

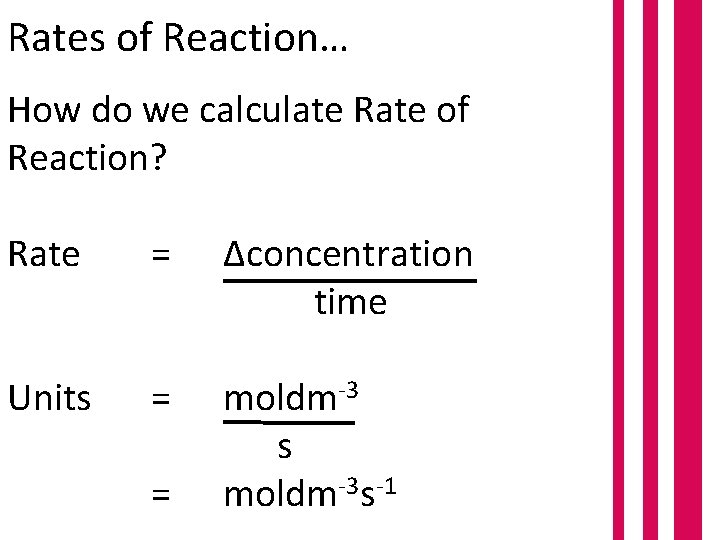
Rates of Reaction… How do we calculate Rate of Reaction? Rate = Δconcentration time Units = moldm-3 s moldm-3 s-1 =

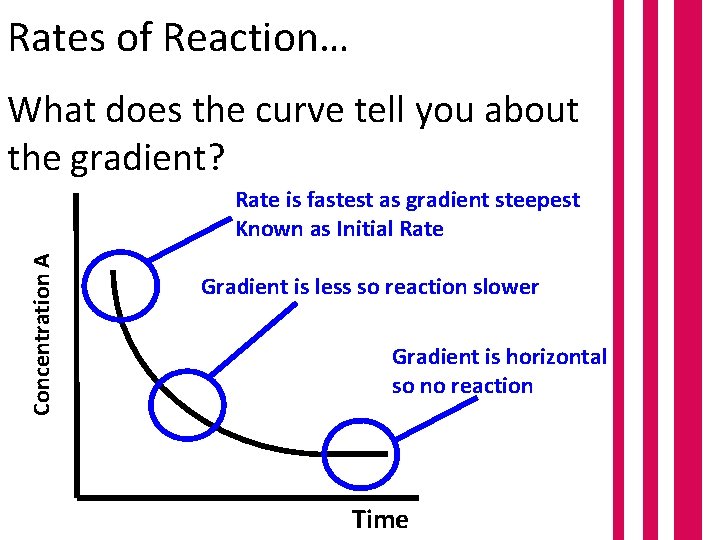
Rates of Reaction… What does the curve tell you about the gradient? Concentration A Rate is fastest as gradient steepest Known as Initial Rate Gradient is less so reaction slower Gradient is horizontal so no reaction Time

Rates of Reaction… Draw the graph showing the change in concentration of C and label: • Fastest rate of reaction • Where reaction has stopped

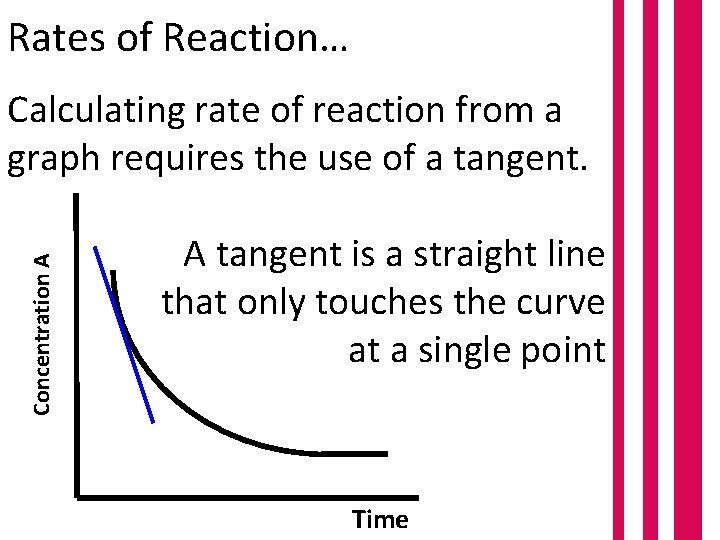
Rates of Reaction… Concentration A Calculating rate of reaction from a graph requires the use of a tangent. A tangent is a straight line that only touches the curve at a single point Time

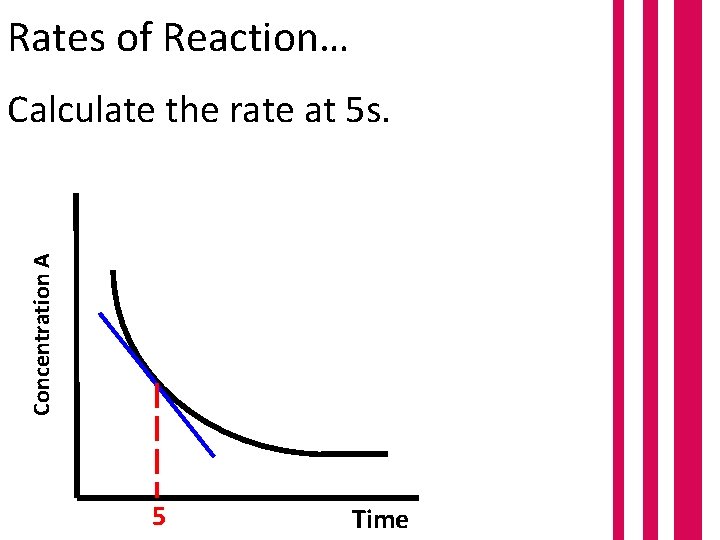
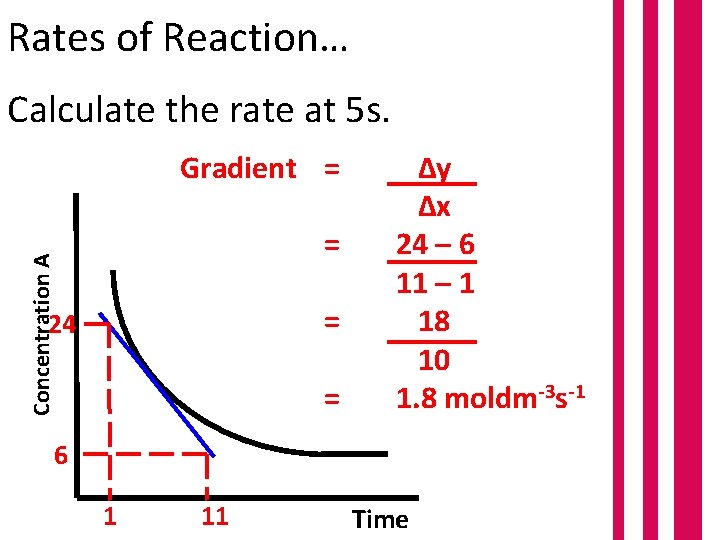
Rates of Reaction… Concentration A Calculate the rate at 5 s. 5 Time

Rates of Reaction… Calculate the rate at 5 s. Gradient = Concentration A = = 24 = Δy Δx 24 – 6 11 – 1 18 10 1. 8 moldm-3 s-1 6 1 11 Time

Rates of Reaction… How do we get the results to produce a graph? • Concentration burette • Gas Volume gas syringe • Mass balance

Rates of Reaction… Back to GCSE! What are the factors affecting rate of reaction? • • Temperature Concentration Pressure Surface Area

Rates of Reaction… But these only increase the number of collisions per unit time. What are the two requirements for a reaction to take place? • Activation energy met • Orientation of particles

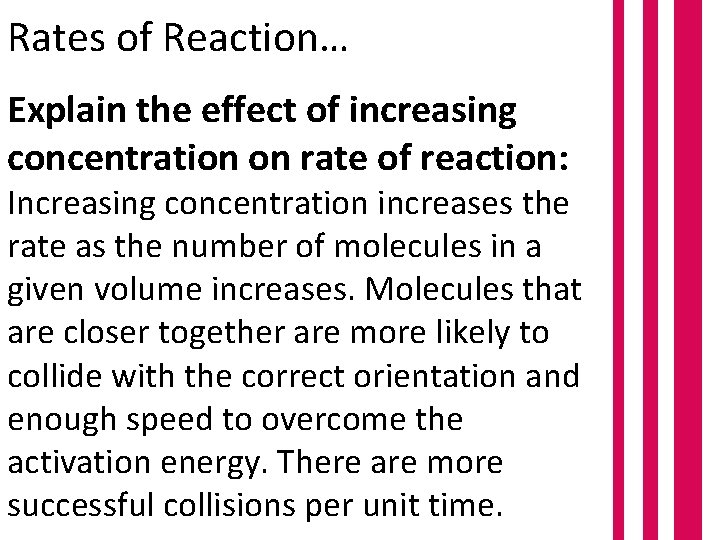
Rates of Reaction… Explain the effect of increasing concentration on rate of reaction: Increasing concentration increases the rate as the number of molecules in a given volume increases. Molecules that are closer together are more likely to collide with the correct orientation and enough speed to overcome the activation energy. There are more successful collisions per unit time.

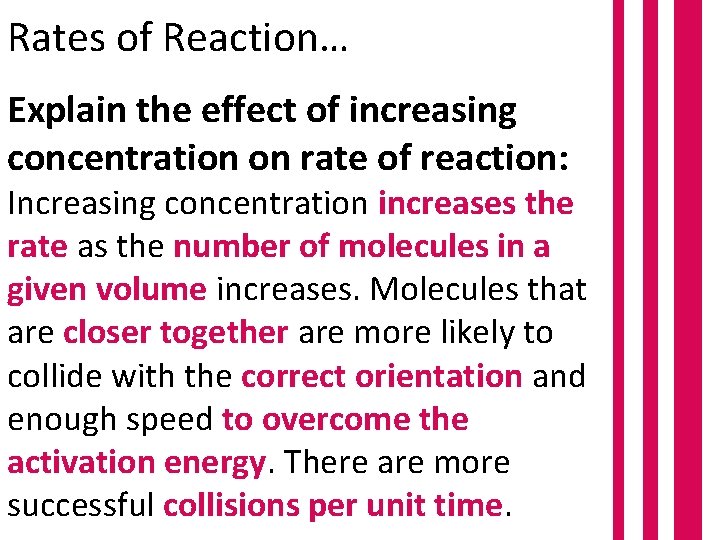
Rates of Reaction… Explain the effect of increasing concentration on rate of reaction: Increasing concentration increases the rate as the number of molecules in a given volume increases. Molecules that are closer together are more likely to collide with the correct orientation and enough speed to overcome the activation energy. There are more successful collisions per unit time.

Rates of Reaction… Your turn! Explain the effect of adding a catalyst to the rate of reaction: Increase rate Lowers the activation energy By offering an alternate pathway More particles have enough energy to overcome lower Ea • Correct orientation • More successful collisions /unit time • •

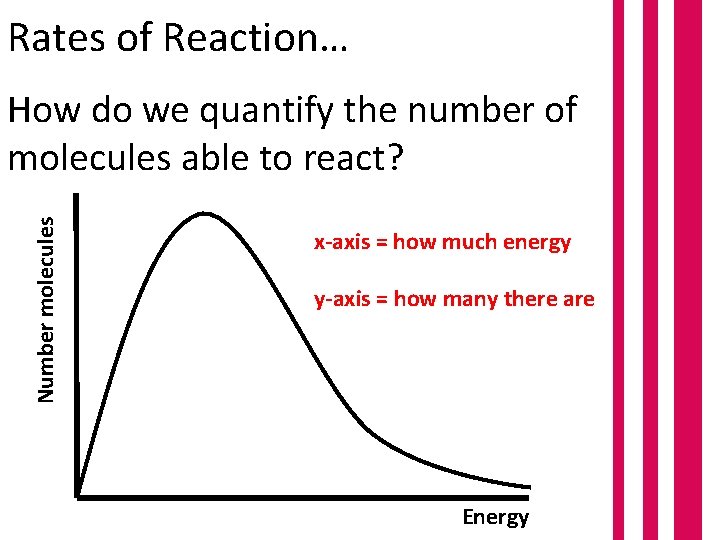
Rates of Reaction… Number molecules How do we quantify the number of molecules able to react? x-axis = how much energy y-axis = how many there are Energy

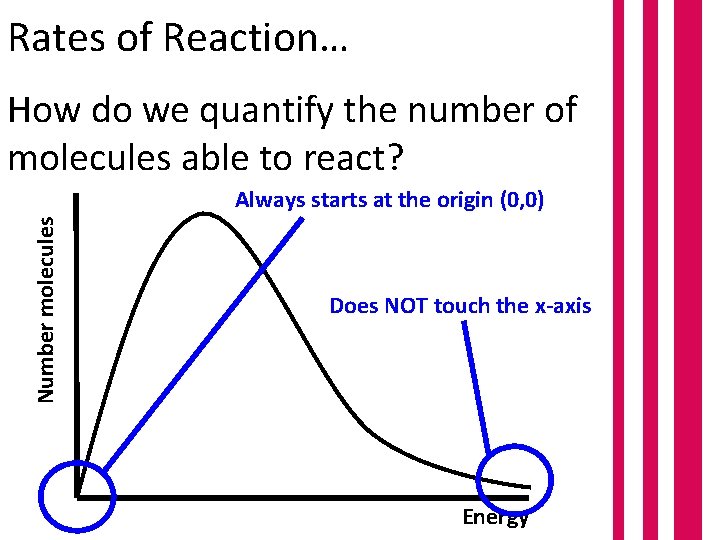
Rates of Reaction… How do we quantify the number of molecules able to react? Number molecules Always starts at the origin (0, 0) Does NOT touch the x-axis Energy

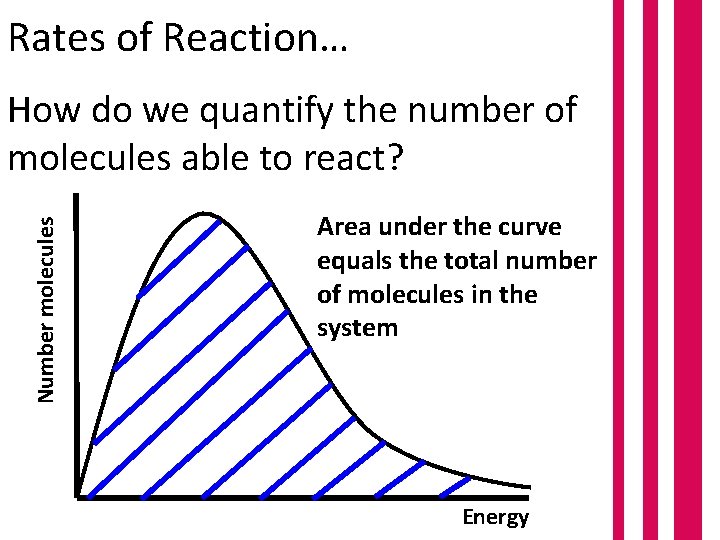
Rates of Reaction… Number molecules How do we quantify the number of molecules able to react? Area under the curve equals the total number of molecules in the system Energy

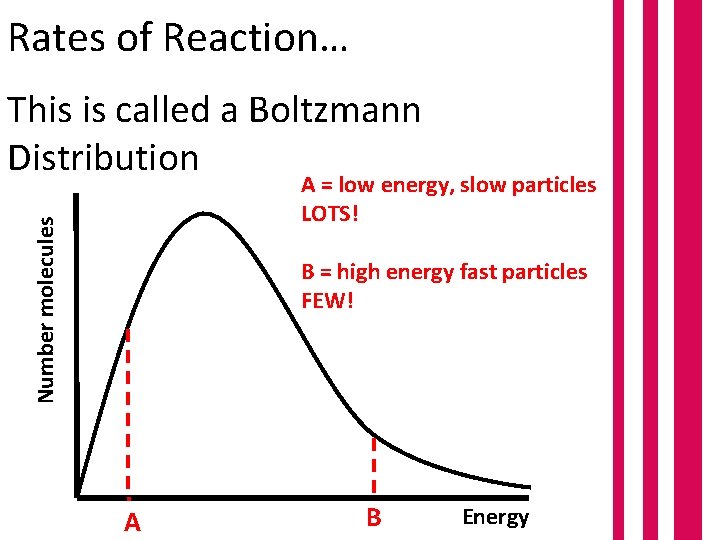
Rates of Reaction… This is called a Boltzmann Distribution Number molecules A = low energy, slow particles LOTS! B = high energy fast particles FEW! A B Energy

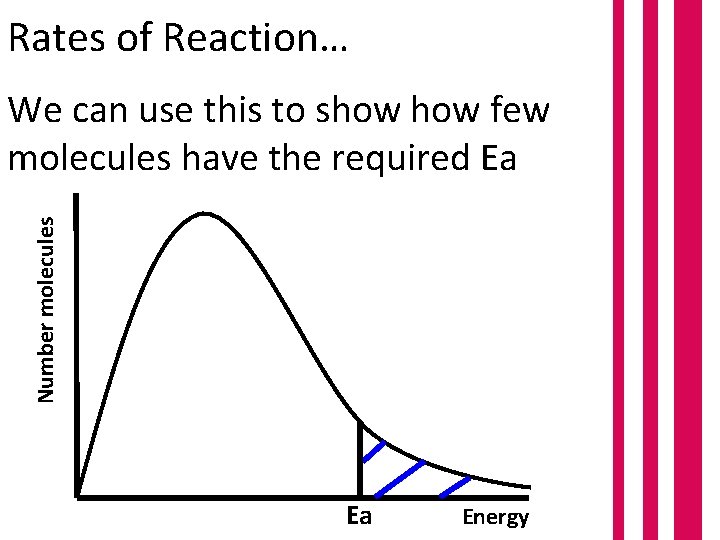
Rates of Reaction… Number molecules We can use this to show few molecules have the required Ea Ea Energy

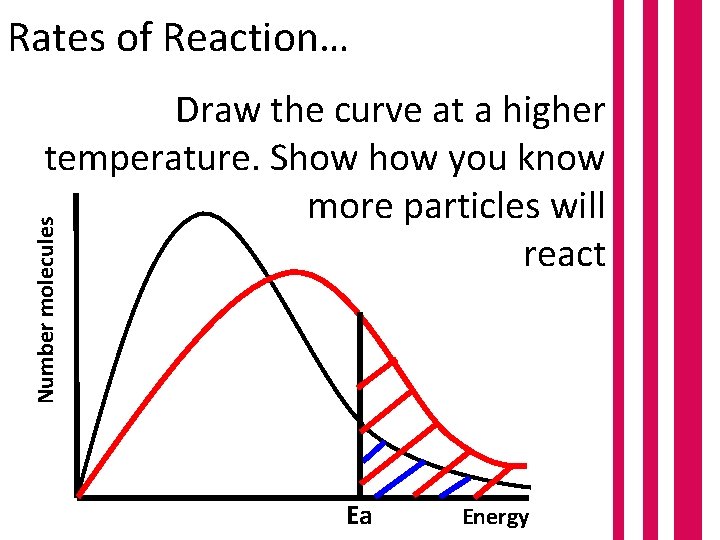
Rates of Reaction… Number molecules Draw the curve at a higher temperature. Show you know more particles will react Ea Energy

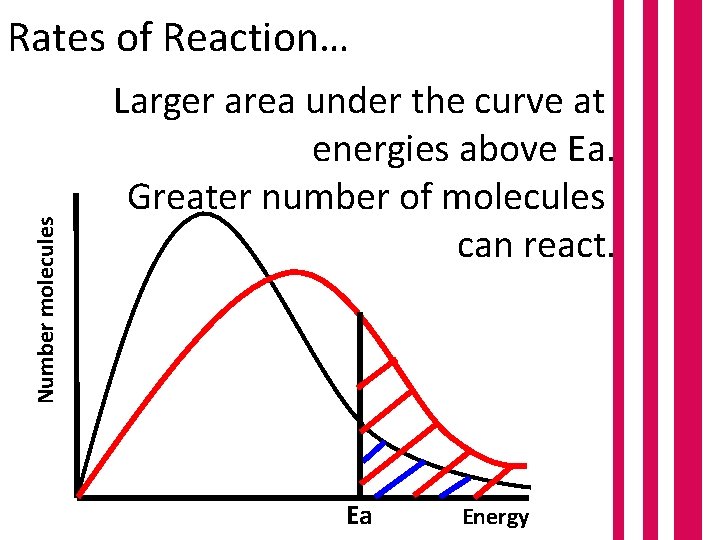
Number molecules Rates of Reaction… Larger area under the curve at energies above Ea. Greater number of molecules can react. Ea Energy

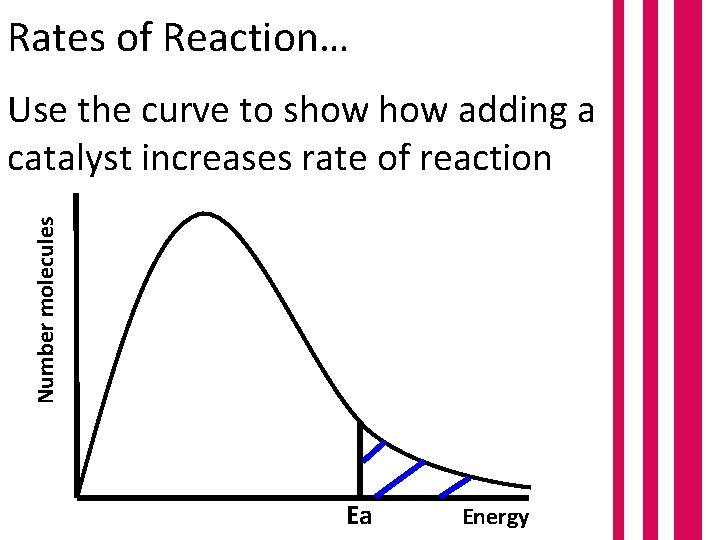
Rates of Reaction… Number molecules Use the curve to show adding a catalyst increases rate of reaction Ea Energy

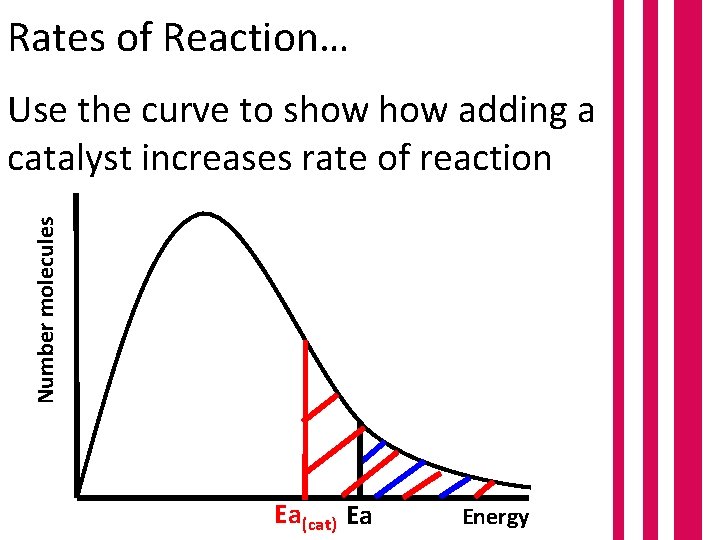
Rates of Reaction… Number molecules Use the curve to show adding a catalyst increases rate of reaction Ea(cat) Ea Energy

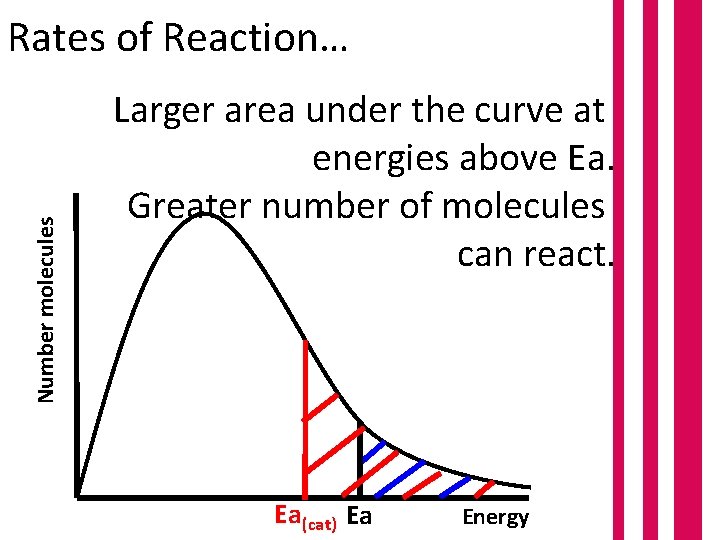
Number molecules Rates of Reaction… Larger area under the curve at energies above Ea. Greater number of molecules can react. Ea(cat) Ea Energy

Rates of Reaction… How do we calculate Rate of Reaction?

Rates of Reaction… How do we calculate Rate of Reaction?

Rates of Reaction… How do we calculate Rate of Reaction?