Rates of Reaction Equilibrium Introduction to Rate Rate
Rates of Reaction & Equilibrium Introduction to Rate
Rate of Reaction: ¡ the change in “something” of the reactants or products over time or per unit time l l l ¡ mass or moles over time** concentration over time color over time the rate of a reaction can be fast, slow or zero
Chemical Reactions ¡ are the result of collisions between atoms, ions or molecules Cl Na ¡ involve bond breaking (releases energy) ¡ involve bond making (requires energy)
Collision Theory r u c oc ¡ explains why reactions have differentnrates t ’ o d s n o i t s c d a n e r o b e p 1. the reacting particles must collide m m m r o e s o t f y m & h o k w o a r s e t n r i a b a l o t y Exp g r e 2. thegparticles must have enough energy en h u o n e for the breaking & making of bonds t o N 3. the colliding particles must collide at the right orientation or geometry
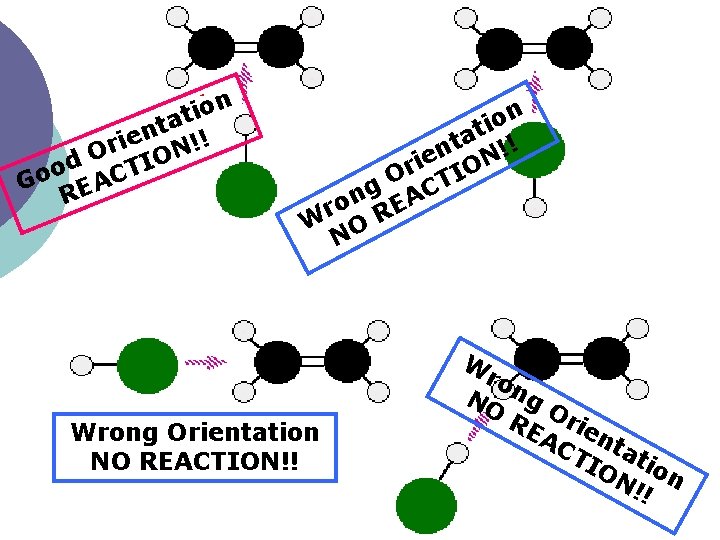
n o i t a t ien N!! r O IO d o T Go EAC R n o i at !! t n N e i r IO O T g AC n ro RE W O N Wrong Orientation NO REACTION!! W ro NO ng RE Orie AC nt TI atio ON n !!
Activation Energy ¡ the min amount of energy that colliding particles must have in order to react l “barrier” or “hurdle” reactants must overcome
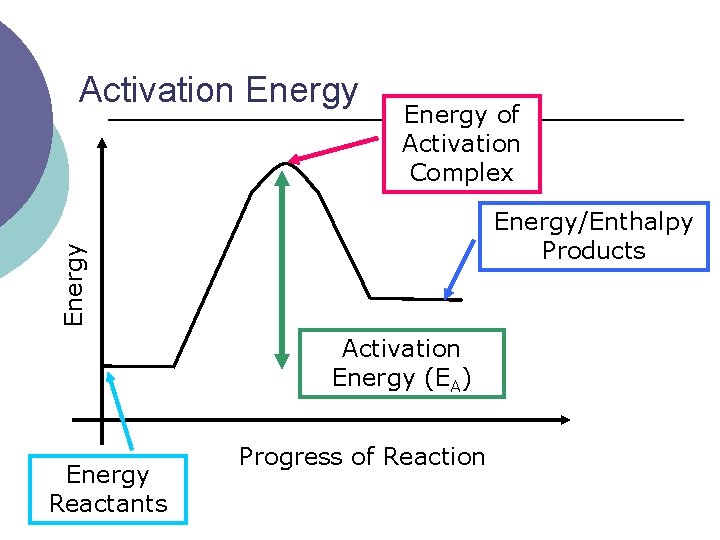
Activation Energy of Activation Complex Energy/Enthalpy Products Activation Energy (EA) Energy Reactants Progress of Reaction
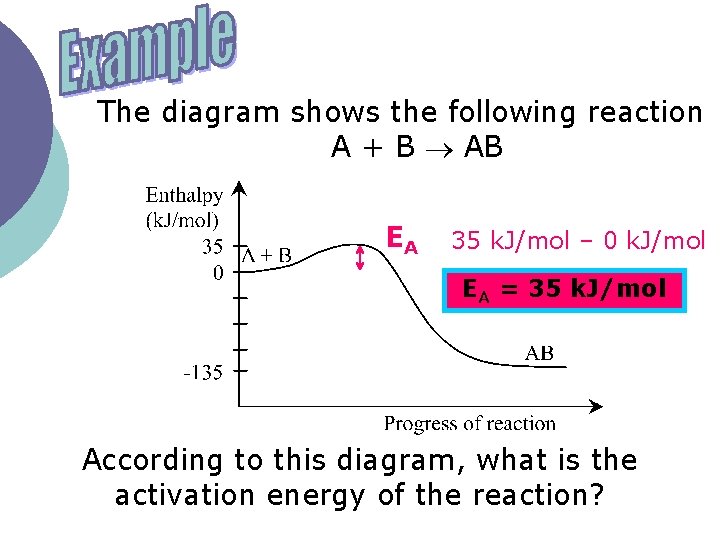
The diagram shows the following reaction A + B AB EA 35 k. J/mol – 0 k. J/mol EA = 35 k. J/mol According to this diagram, what is the activation energy of the reaction?
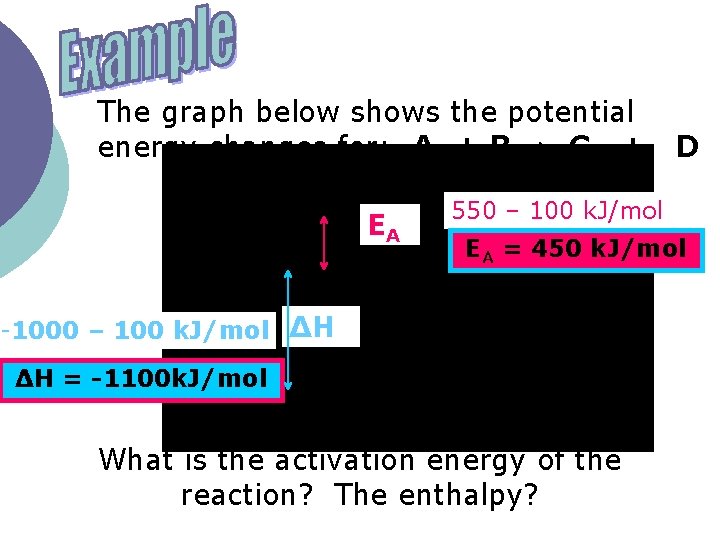
The graph below shows the potential energy changes for: A + B C + EA D 550 – 100 k. J/mol EA = 450 k. J/mol -1000 – 100 k. J/mol ∆H ∆H = -1100 k. J/mol What is the activation energy of the reaction? The enthalpy?
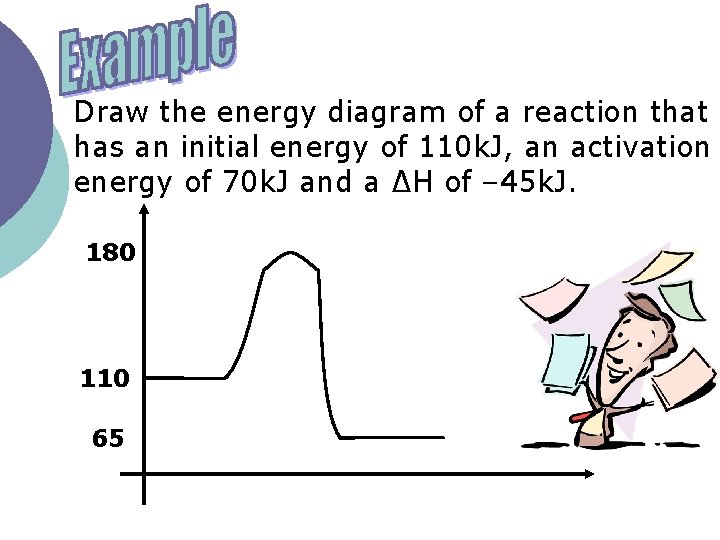
Draw the energy diagram of a reaction that has an initial energy of 110 k. J, an activation energy of 70 k. J and a ∆H of – 45 k. J. 180 110 65
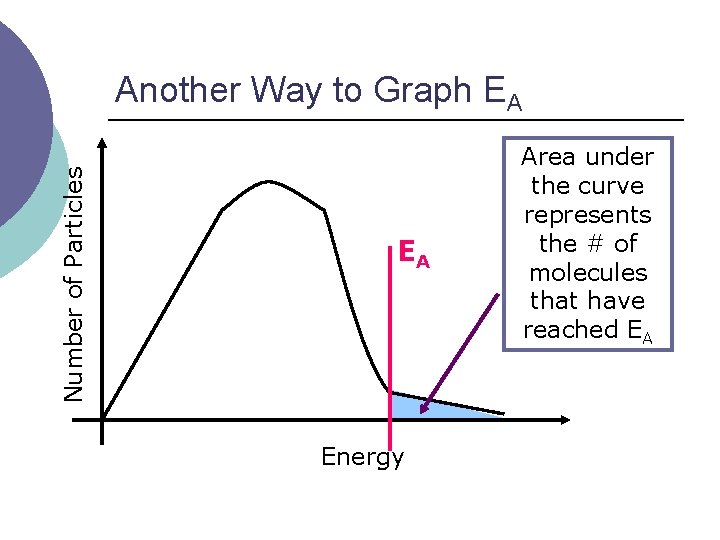
Number of Particles Another Way to Graph EA EA Energy Area under the curve represents the # of molecules that have reached EA
- Slides: 11