Rates of Reaction and Particle Size What affects













- Slides: 27

Rates of Reaction and Particle Size What affects the rate of a reaction? Starter: How can I make sugar dissolve faster in water?

Success criteria • Must – I can name 5 things that can affect the rate of a reaction. • Should – I can do practical investigations to find out about rates of reaction. • Could – I can write equations to show my reactions

Rates of reaction Chemical reactions occur when particles of reactant collide with enough energy to react.

What can affect how quickly collisions happen?

Particle Size • Will a sugar lump or a tea spoon of sugar dissolve faster?

Demo 1: Burning magnesium • What is the difference between burning powdered magnesium and a piece of magnesium?

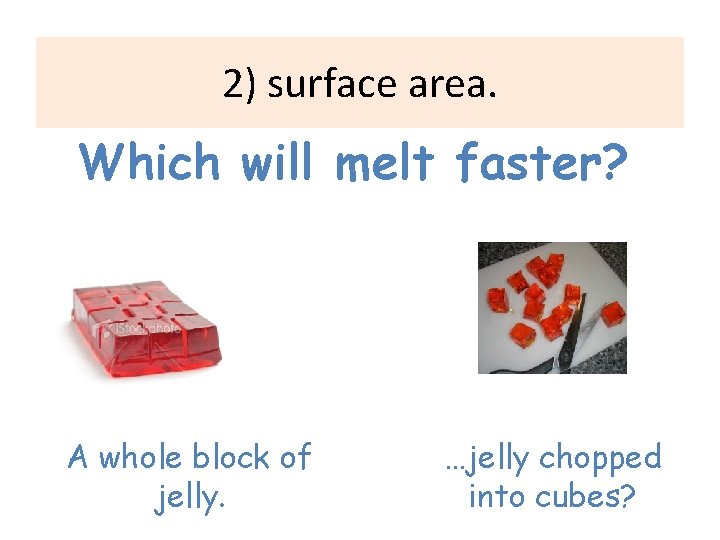
2) surface area. Which will melt faster? A whole block of jelly. …jelly chopped into cubes?

Why do they dissolve faster? Because if you compare a the whole jelly with the small pieces, the small pieces have a LARGER SURFACE AREA

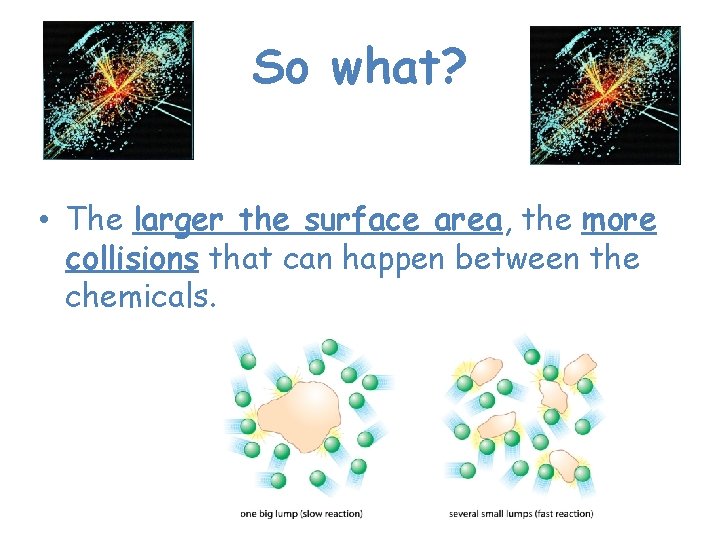
So what? • The larger the surface area, the more collisions that can happen between the chemicals.

Temperature • Will chemicals react faster when it is hot or cold?

Results • You will ALL do 0, room temperature and 40 degrees. • Each group will use a different method and report back. • WARNING: hot acid is extra dangerous! GOGGLES ON

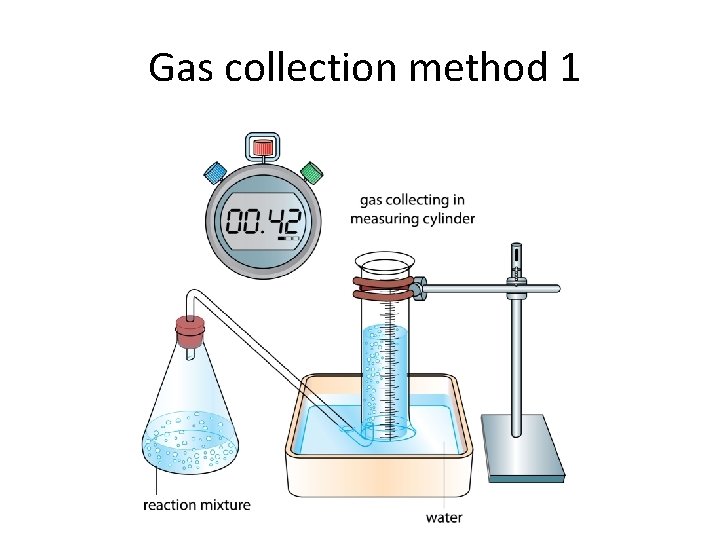
Gas collection method 1

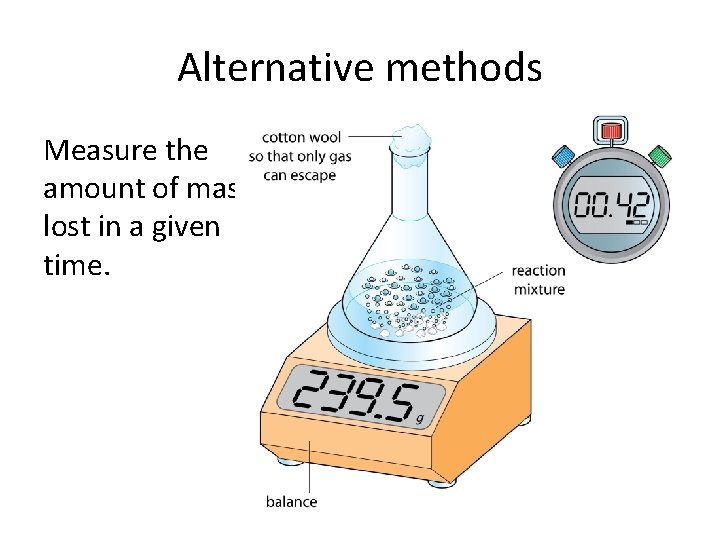
Alternative methods Measure the amount of mass lost in a given time.

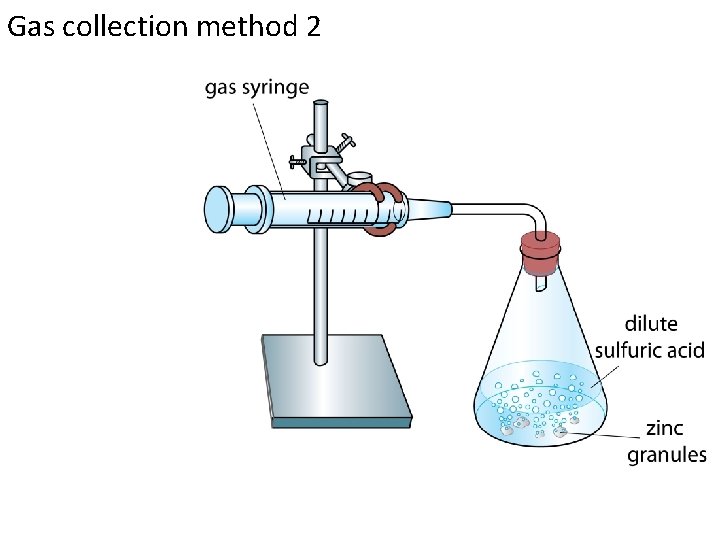
Gas collection method 2

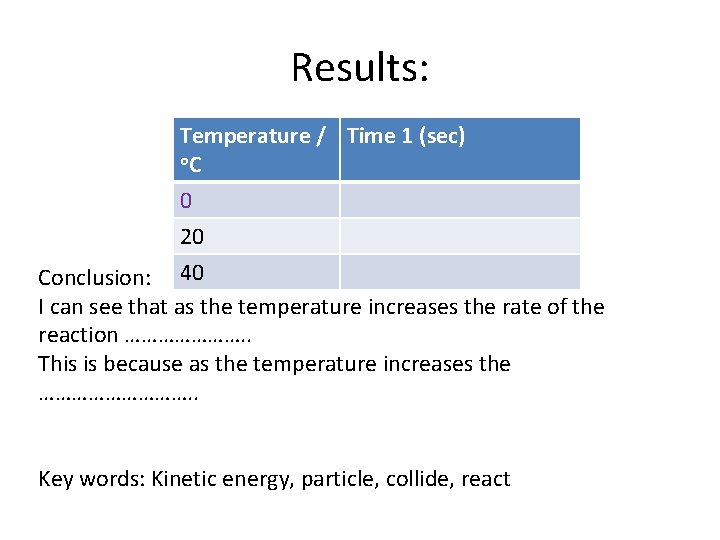
Results: Temperature / Time 1 (sec) o. C 0 20 Conclusion: 40 I can see that as the temperature increases the rate of the reaction …………………. . This is because as the temperature increases the ……………. . Key words: Kinetic energy, particle, collide, react

3) What is a catalyst? • A catalyst is a chemical that speeds up a reaction without being used up.

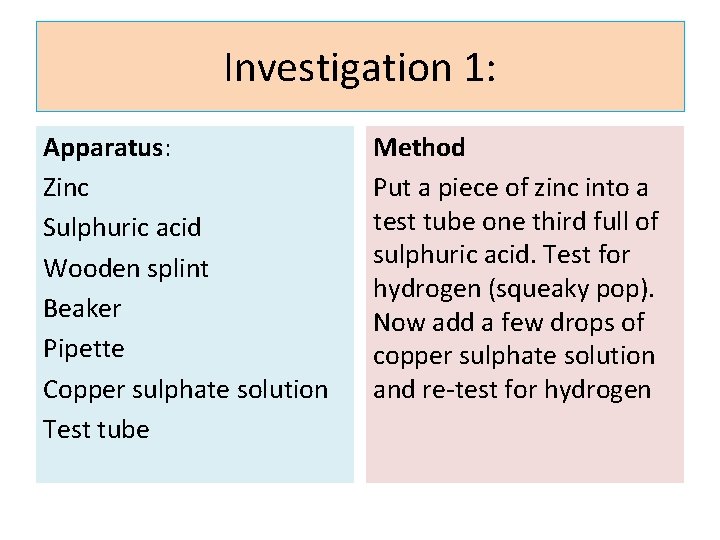
Investigation 1: Apparatus: Zinc Sulphuric acid Wooden splint Beaker Pipette Copper sulphate solution Test tube Method Put a piece of zinc into a test tube one third full of sulphuric acid. Test for hydrogen (squeaky pop). Now add a few drops of copper sulphate solution and re-test for hydrogen

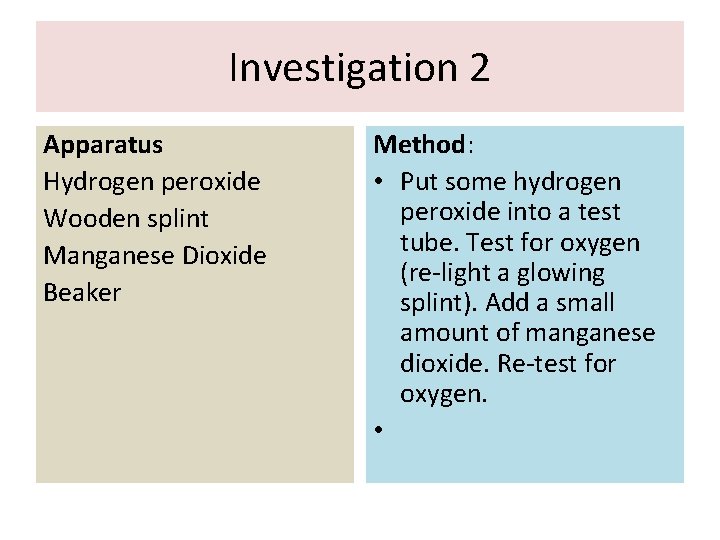
Investigation 2 Apparatus Hydrogen peroxide Wooden splint Manganese Dioxide Beaker Method: • Put some hydrogen peroxide into a test tube. Test for oxygen (re-light a glowing splint). Add a small amount of manganese dioxide. Re-test for oxygen. •

Plenary • Can you think of any other situations at home where surface area has an effect on something.

Rate of Reaction and concentration How does concentration affect the rate of reaction? Starter: What can happen when particles collide?

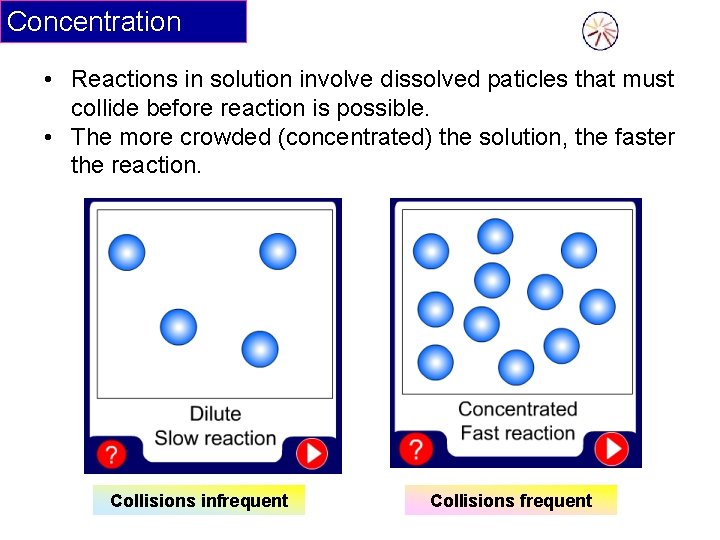
Concentration • Reactions in solution involve dissolved paticles that must collide before reaction is possible. • The more crowded (concentrated) the solution, the faster the reaction. Collisions infrequent Collisions frequent

Lets investigate AIM: to investigate how concentration affects the rate of a reaction. Follow the instructions on the worksheet to find out how the concentration of sodium thiosulphate affects the rate of a reaction.

Results • Complete a line graph of your results

Concentration and Rates • The rate of a reaction will increase with an increase in concentration because there are more particles. • The more particles there are, the more reactions will take place

Plenary • Produce a revision card for each factor. 1) Catalysts 2) Particle size 3) Concentration 4) Temperature Draw a graph for each.

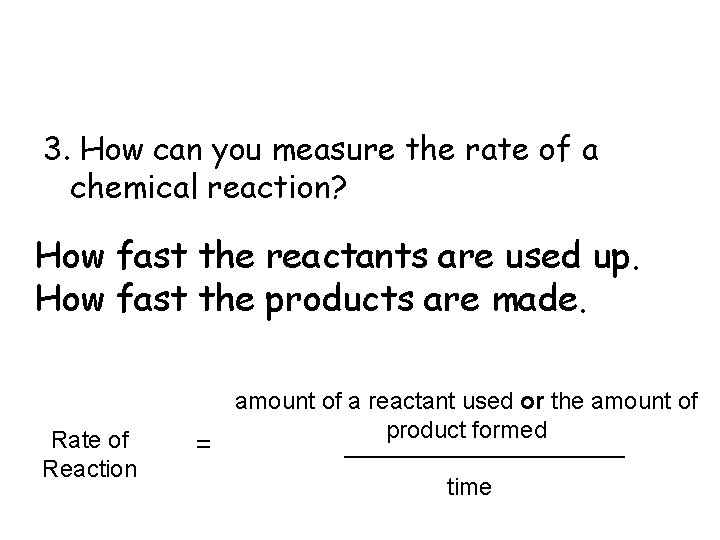
3. How can you measure the rate of a chemical reaction? How fast the reactants are used up. How fast the products are made. Rate of Reaction amount of a reactant used or the amount of product formed ___________ = time
