Rate of Visual Field Progression in Eyes With

- Slides: 11

Rate of Visual Field Progression in Eyes With Optic Disc Hemorrhages in the Ocular Hypertension Treatment Study De Moraes CG, Demirel S, Gardiner SK, et al; Ocular Hypertension Treatment Study Group. Rate of visual field progression in eyes with optic disc hemorrhages in the Ocular Hypertension Treatment Study. Arch Ophthalmol. Published online August 13, 2012. doi: 10. 1001/archophthalmol. 2012. 2324. Copyright restrictions may apply

Introduction • Optic disc hemorrhage (DH) is an important risk factor for glaucoma onset and progression. • The Ocular Hypertension Treatment Study (OHTS) showed that eyes experiencing DH have a 3 -fold increased risk of conversion to glaucoma. • Objectives: – To compare rates of visual field (VF) change in ocular hypertensive eyes with and without DH in the OHTS. – To compare whether treated and untreated eyes had different rates of DH detection. Copyright restrictions may apply

Methods • Study Design: Randomized clinical trial. • Participants: OHTS participants who had a minimum of 10 reliable VF tests and were followed up for at least 5 years. Copyright restrictions may apply

Methods • Data Analysis: Trend analyses of VF sequences over time of DH and non. DH eyes were assessed by regression of mean deviation (MDR) and pointwise linear regression (PLR). • Limitations: – All VFs (before and after DH detection) were included when calculating the rates of VF change. – Patients had optic disc photographs taken every 12 months, and DH eyes may have been missed. – The group with DH had more risk factors for progression at baseline than the group without DH. Copyright restrictions may apply

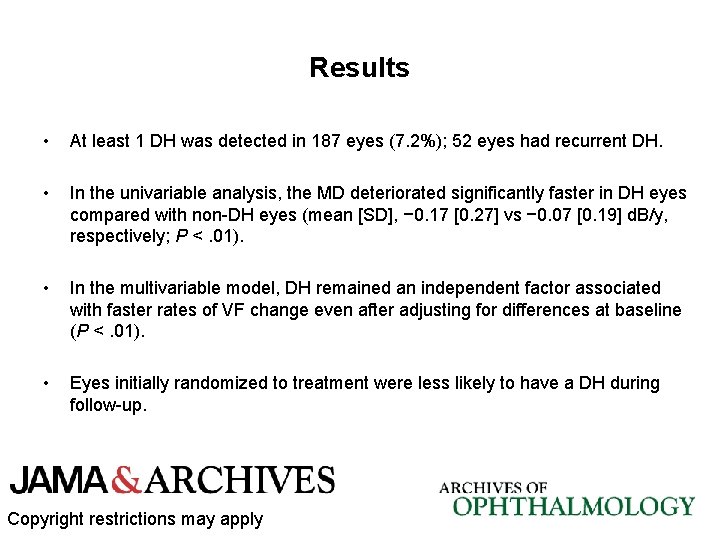
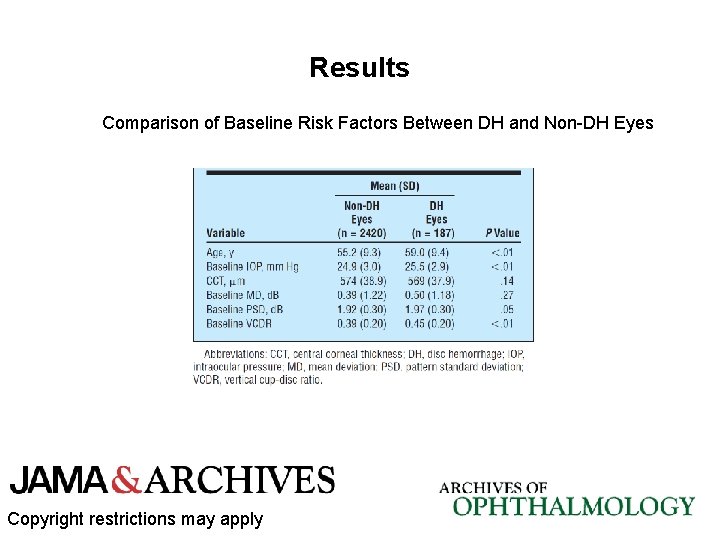
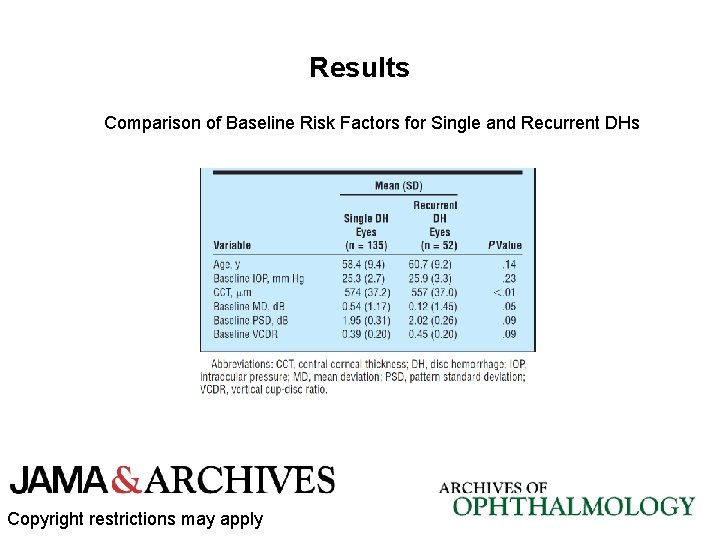
Results • At least 1 DH was detected in 187 eyes (7. 2%); 52 eyes had recurrent DH. • In the univariable analysis, the MD deteriorated significantly faster in DH eyes compared with non-DH eyes (mean [SD], − 0. 17 [0. 27] vs − 0. 07 [0. 19] d. B/y, respectively; P <. 01). • In the multivariable model, DH remained an independent factor associated with faster rates of VF change even after adjusting for differences at baseline (P <. 01). • Eyes initially randomized to treatment were less likely to have a DH during follow-up. Copyright restrictions may apply

Results Comparison of Baseline Risk Factors Between DH and Non-DH Eyes Copyright restrictions may apply

Results Comparison of Baseline Risk Factors for Single and Recurrent DHs Copyright restrictions may apply

Results Multivariable Model: Association Between Baseline Variables, DH, and the Global Rate of VF Change (Mean Deviation Rate) Copyright restrictions may apply

Comment • The presence of DH affects clinical outcomes and should alert the physician to an increased patient risk profile that may require more aggressive therapy. • Recurrent DH does not appear to affect the global rate of VF change as captured by MDR but may impact localized rates as captured via PLR. • Initial randomization to treatment significantly decreased the risk of developing a DH compared with eyes in the observation group, while the previous OHTS report found no statistical significance between groups. Copyright restrictions may apply

Comment • Both the mechanism of DH development and why it leads to faster progression need to be elucidated. • New models for risk calculation in patients with ocular hypertension might benefit from inclusion of DH as a risk factor. • Assuming linearity of VF change, DH eyes will take half as long to reach VF sensitivity values consistent with meaningful visual impairment. • Frequent optic disc photography is mandatory for monitoring and risk assessment in patients with ocular hypertension. Copyright restrictions may apply

Contact Information • If you have questions, please contact the corresponding author: – Carlos Gustavo De Moraes, MD, 310 E 14 th St, New York, NY 10003 (demoraesmd@gmail. com). Funding/Support • This work was supported by National Institutes of Health grants EY 09307 and EY 09341; the National Center on Minority Health and Health Disparities; Research to Prevent Blindness; the Edith C. Blum Foundation Research Fund of the New York Glaucoma Research Institute; Legacy Good Samaritan Foundation; Merck Inc; and Pfizer Inc. Copyright restrictions may apply