Rate of Reactions Examples 1 The following reaction












![2. a) From the equation stoichiometry, Δ[H 2 O] = 6/2 Δ[N 2], so 2. a) From the equation stoichiometry, Δ[H 2 O] = 6/2 Δ[N 2], so](https://slidetodoc.com/presentation_image_h/22c17874a66a723cd084de9f2ae95e46/image-17.jpg)








- Slides: 27

Rate of Reactions Examples

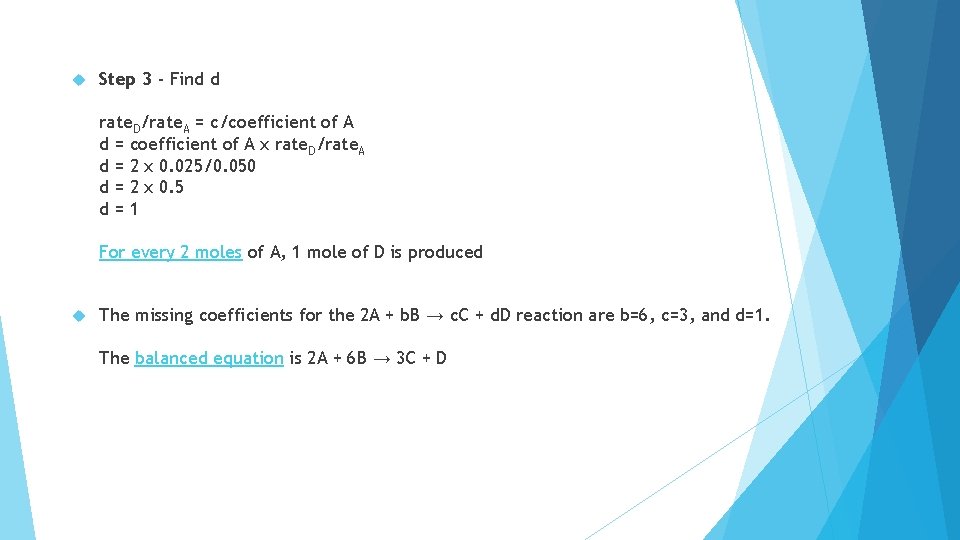
1. The following reaction is observed: 2 A + b. B → c. C + d. D As the reaction progressed, the concentrations changed by these rates rate. A = 0. 050 mol/L·s rate. B = 0. 150 mol/L·s rate. C = 0. 075 mol/L·s rate. D = 0. 025 mol/L·s What are the values for the coefficients b, c, and d?

2. For the oxidation of ammonia 4 NH 3 +3 O 2→ 2 N 2 +6 H 2 O it was found that the rate of formation of N 2 was 0. 27 mol L– 1 s– 1. a) At what rate was water being formed? b) At what rate was ammonia being consumed?

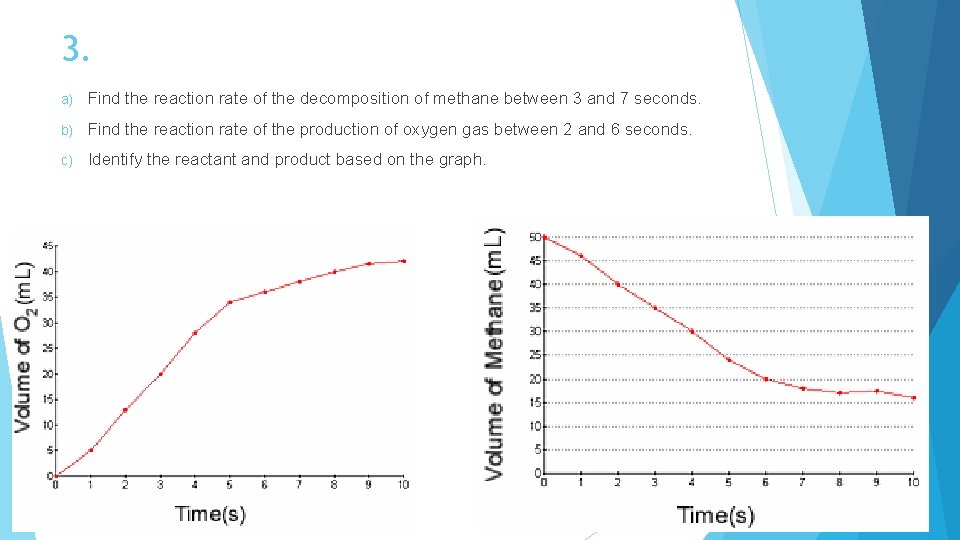
3. a) Find the reaction rate of the decomposition of methane between 3 and 7 seconds. b) Find the reaction rate of the production of oxygen gas between 2 and 6 seconds. c) Identify the reactant and product based on the graph.

4. The rate of a chemical reaction can be expressed in a) energy released per mole of reactant b) Grams per mole of reactant c) moles per liter of solution d) Volume of gas per minute

5. Consider the following reaction Ca. O (s) + 2 HCl (aq) → Ca. Cl 2 (aq) + H 2 O (l) Which of the following could be used to measure the rate of the reaction? a) Measure Δpressure/Δtime b) Measure Δtotal mass/Δtime c) Measure Δp. H/Δtime d) Measure Δvolume/Δtime

6. Consider the following reaction N 2 H 4 (l)+2 H 2 O 2 (l)→N 2 (g)+4 H 2 O(l) Which of the following could be used to determine the reaction rate in a closed system. a) Measure the increase in gas pressure b) Measure the decrease in gas pressure c) measure the increase in mass of the system d) Measure the decrease in mass of the system

7. Consider the following reaction N 2 H 4 (l) + 2 H 2 O 2 (l) → N 2 (g) + 4 H 2 O(l) In 55. 0 sec, 0. 165 mole of H 2 O 2 is consumed. The rate of production of N 2 is a) 1. 5 x 10 -3 mole in 55 s b) 0. 33 mole per second c) 1. 5 x 10 -3 mole per second d) 0. 006 mole per second

8. Which factor explains why lithium metal generally reacts faster than gold metal? a) Concentration b) Nature c) of the substance Surface area d) Temperature

9. Consider the following reaction N 2 (g)+2 H 2 (g) → 2 NH 3 (g) The rate of formation of NH 3 is 0. 0153 g/s, the rate of consumption of N 2 is a) 0. 0765 g/s b) 0. 0306 g/s c) 0. 000449 mole/s d) 0. 00180 mole/s

10. Nitrogen monoxide and hydrogen react according to the following reaction. 2 NO(g) + 2 H 2 (g) → N 2 (g) + 2 H 2 O(g) If the rate of hydrogen consumption is 0. 87 g in 10 minutes, what is the rate of nitrogen production? a) 0. 044 g/min b) 0. 61 g c) in 10 minutes 1. 2 g/min d) 0. 61 g/min

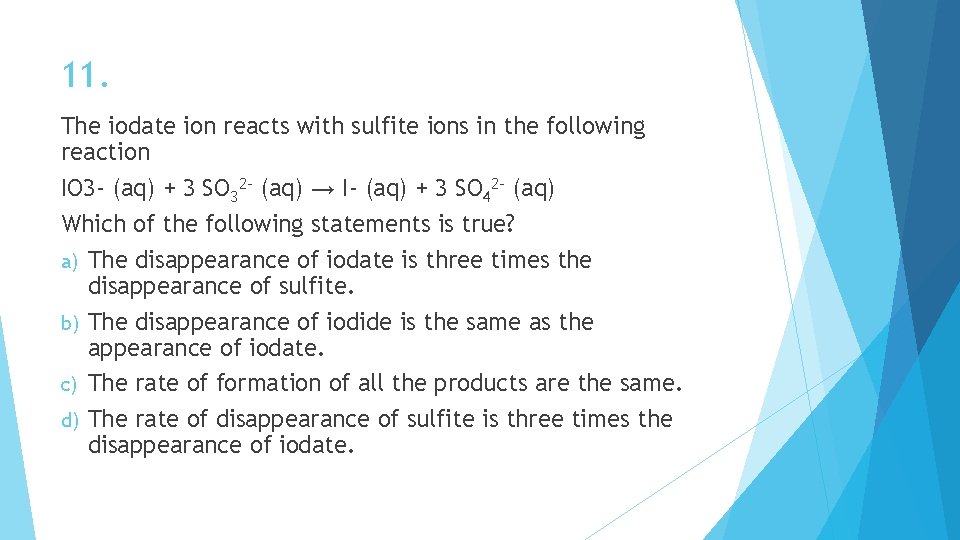
11. The iodate ion reacts with sulfite ions in the following reaction IO 3 - (aq) + 3 SO 32 - (aq) → I- (aq) + 3 SO 42 - (aq) Which of the following statements is true? a) The disappearance of iodate is three times the disappearance of sulfite. b) The disappearance of iodide is the same as the appearance of iodate. c) The rate of formation of all the products are the same. d) The rate of disappearance of sulfite is three times the disappearance of iodate.

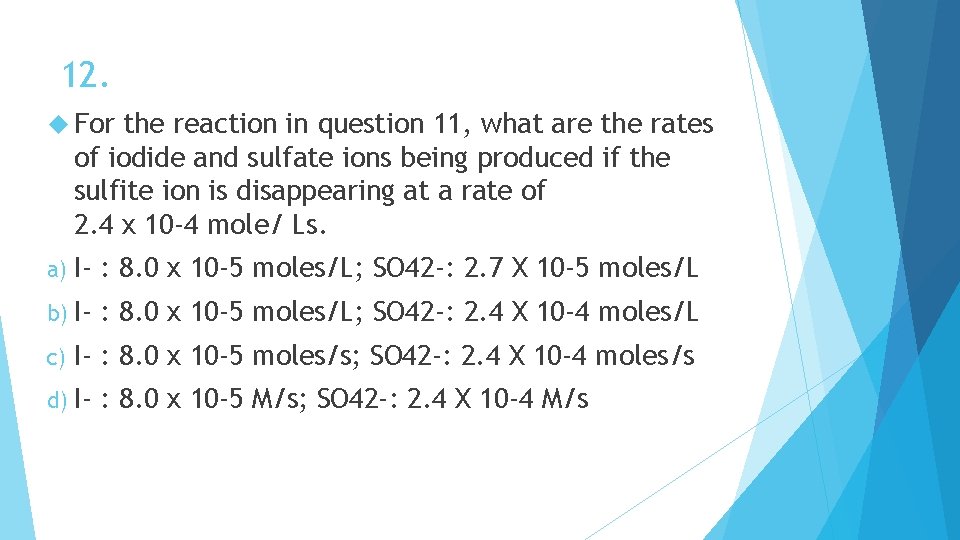
12. For the reaction in question 11, what are the rates of iodide and sulfate ions being produced if the sulfite ion is disappearing at a rate of 2. 4 x 10 -4 mole/ Ls. a) I- : 8. 0 x 10 -5 moles/L; SO 42 -: 2. 7 X 10 -5 moles/L b) I- : 8. 0 x 10 -5 moles/L; SO 42 -: 2. 4 X 10 -4 moles/L c) I- : 8. 0 x 10 -5 moles/s; SO 42 -: 2. 4 X 10 -4 moles/s d) I- : 8. 0 x 10 -5 M/s; SO 42 -: 2. 4 X 10 -4 M/s

Answers

1. Step 1 - Find b rate. B/rate. A = b/coefficient of A b = coefficient of A x rate. B/rate. A b = 2 x 0. 150/0. 050 b=2 x 3 b=6 For every 2 moles of A, 6 moles of B are needed to complete the reaction Step 2 - Find c rate. B/rate. A = c/coefficient of A c = coefficient of A x rate. C/rate. A c = 2 x 0. 075/0. 050 c = 2 x 1. 5 c=3 For every 2 moles of A, 3 moles of C are produced

Step 3 - Find d rate. D/rate. A = c/coefficient of A d = coefficient of A x rate. D/rate. A d = 2 x 0. 025/0. 050 d = 2 x 0. 5 d=1 For every 2 moles of A, 1 mole of D is produced The missing coefficients for the 2 A + b. B → c. C + d. D reaction are b=6, c=3, and d=1. The balanced equation is 2 A + 6 B → 3 C + D
![2 a From the equation stoichiometry ΔH 2 O 62 ΔN 2 so 2. a) From the equation stoichiometry, Δ[H 2 O] = 6/2 Δ[N 2], so](https://slidetodoc.com/presentation_image_h/22c17874a66a723cd084de9f2ae95e46/image-17.jpg)
2. a) From the equation stoichiometry, Δ[H 2 O] = 6/2 Δ[N 2], so the rate of formation of H 2 O is 3 × (0. 27 mol L– 1 s– 1) = 0. 81 mol L– 1 s– 1. b) 4 moles of NH 3 are consumed for every 2 moles of N 2 formed, so the rate of disappearance of ammonia is 2 × (0. 27 mol L– 1 s– 1) = 0. 54 mol L– 1 s– 1.

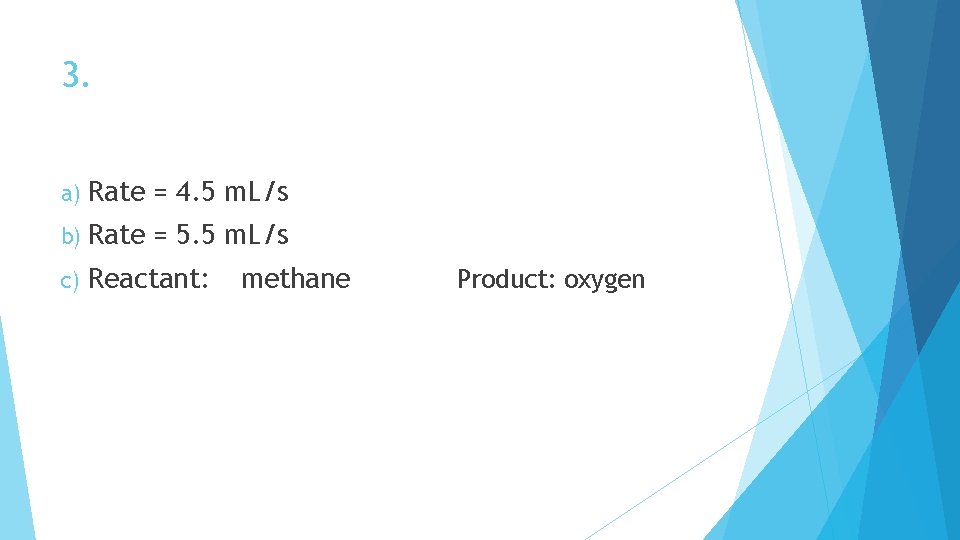
3. a) Rate = 4. 5 m. L/s b) Rate = 5. 5 m. L/s c) Reactant: methane Product: oxygen

4. The rate of a chemical reaction can be expressed in a) energy released per mole of reactant b) Grams per mole of reactant c) moles per liter of solution d) Volume of gas per minute

5. Consider the following reaction Ca. O (s) + 2 HCl (aq) → Ca. Cl 2 (aq) + H 2 O (l) Which of the following could be used to measure the rate of the reaction? a) Measure Δpressure/Δtime b) Measure Δtotal mass/Δtime c) Measure Δp. H/Δtime d) Measure Δvolume/Δtime

6. Consider the following reaction N 2 H 4 (l)+2 H 2 O 2 (l)→N 2 (g)+4 H 2 O(l) Which of the following could be used to determine the reaction rate in a closed system. a) Measure the increase in gas pressure b) Measure the decrease in gas pressure c) measure the increase in mass of the system d) Measure the decrease in mass of the system

7. Consider the following reaction N 2 H 4 (l) + 2 H 2 O 2 (l) → N 2 (g) + 4 H 2 O(l) In 55. 0 sec, 0. 165 mole of H 2 O 2 is consumed. The rate of production of N 2 is 1. 5 x 10 -3 mole in 55 s b) 0. 33 mole per second c) 1. 5 x 10 -3 mole per second d) 0. 006 mole per second a)

8. Which factor explains why lithium metal generally reacts faster than gold metal? a) Concentration b) Nature of the substance c) Surface area d) Temperature

9. Consider the following reaction N 2 (g)+2 H 2 (g) → 2 NH 3 (g) The rate of formation of NH 3 is 0. 0153 g/s, the rate of consumption of N 2 is a) 0. 00765 g/s b) 0. 0306 g/s c) 0. 000449 mole/s d) 0. 00180 mole/s

10. Nitrogen monoxide and hydrogen react according to the following reaction. 2 NO(g) + 2 H 2 (g) → N 2 (g) + 2 H 2 O(g) If the rate of hydrogen consumption is 0. 87 g in 10 minutes, what is the rate of nitrogen production? a) 0. 044 g/min b) 0. 61 g in 10 minutes c) 1. 2 g/min d) 0. 61 g/min

11. The iodate ion reacts with sulfite ions in the following reaction IO 3 - (aq) + 3 SO 32 - (aq) → I- (aq) + 3 SO 42 - (aq) Which of the following statements is true? a) The disappearance of iodate is three times the disappearance of sulfite. b) The disappearance of iodide is the same as the appearance of iodate. c) The rate of formation of all the products are the same. d) The rate of disappearance of sulfite is three times the disappearance of iodate.

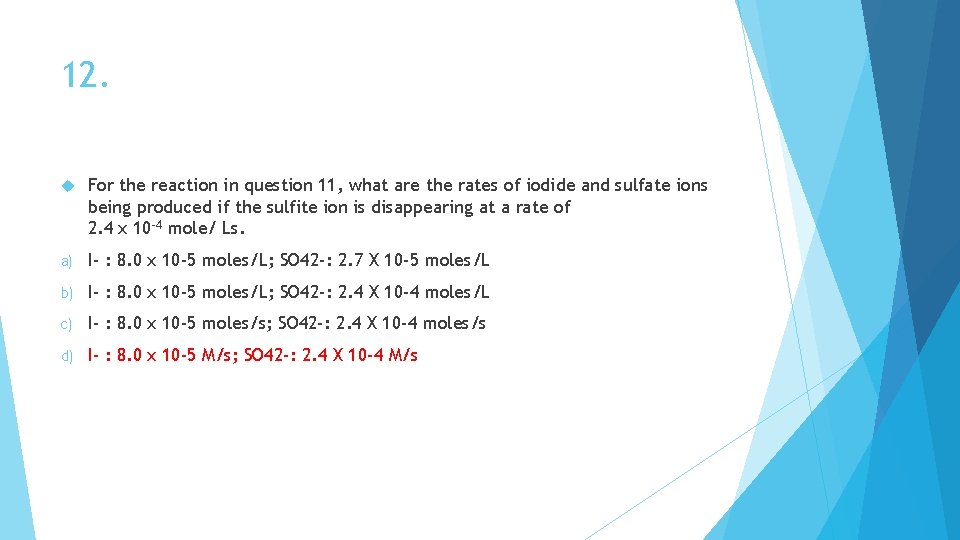
12. For the reaction in question 11, what are the rates of iodide and sulfate ions being produced if the sulfite ion is disappearing at a rate of 2. 4 x 10 -4 mole/ Ls. a) I- : 8. 0 x 10 -5 moles/L; SO 42 -: 2. 7 X 10 -5 moles/L b) I- : 8. 0 x 10 -5 moles/L; SO 42 -: 2. 4 X 10 -4 moles/L c) I- : 8. 0 x 10 -5 moles/s; SO 42 -: 2. 4 X 10 -4 moles/s d) I- : 8. 0 x 10 -5 M/s; SO 42 -: 2. 4 X 10 -4 M/s