Rapid StainFree Western Blotting with the V 3

Rapid Stain-Free Western Blotting with the V 3 Western Workflow™ Biotechnology Explorer. TM Program

Rapid V 3 Stain-Free Western Blotting Workshop Outline § § § § 2 Introduction to Rapid Blotting and V 3 Stain-Free Protein Gel Electrophoresis Stain-Free Gel Activation Western Transfer Stain-Free Gel/Blot Imaging Block nitrocellulose membrane Incubation with antibody solutions Color development of the blot Biotechnology Explorer™ | explorer. bio-rad. com

Rapid Western Blotting + V 3 Stain-Free § A new approach to western blotting workflows – Rapid • Faster electrophoresis times • Faster protein transfer times • Faster protein visualization – Higher Throughput • More gels transferred in a single blotting unit – Real Time Monitoring of Experiment using Stain-Free Technology • Monitor protein separation prior to transfer • Monitor protein transfer prior to blot probing 3 Biotechnology Explorer™ | explorer. bio-rad. com
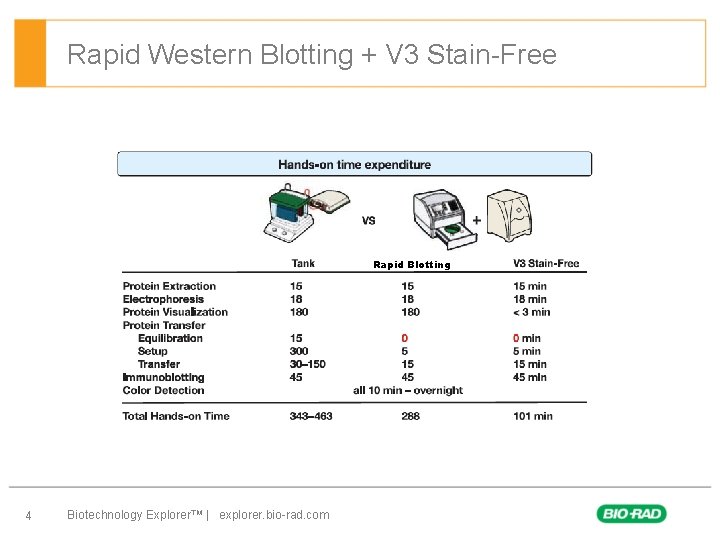
Rapid Western Blotting + V 3 Stain-Free Rapid Blotting 4 Biotechnology Explorer™ | explorer. bio-rad. com
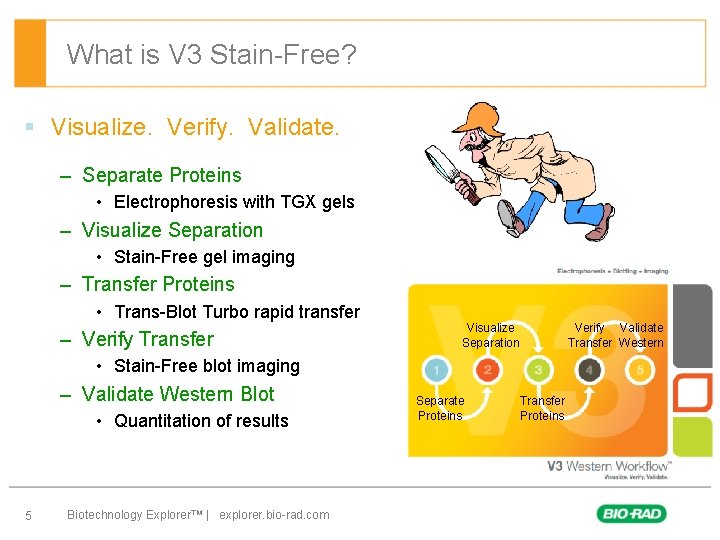
What is V 3 Stain-Free? § Visualize. Verify. Validate. – Separate Proteins • Electrophoresis with TGX gels – Visualize Separation • Stain-Free gel imaging – Transfer Proteins • Trans-Blot Turbo rapid transfer – Verify Transfer Visualize Separation Verify Validate Transfer Western • Stain-Free blot imaging – Validate Western Blot • Quantitation of results 5 Biotechnology Explorer™ | explorer. bio-rad. com Separate Proteins Transfer Proteins
Western Transfer: Blotting Methods § Methods to transfer proteins to solid support – Microfiltration • Used to capture proteins that are in solution, utilizes vacuum for protein immobilization onto membrane • Rapid due to lack of size separation step, but may be less informative – Diffusion/Capillary • Used to transfer proteins from gels, involves wicking of buffer through a weighted transfer stack, is very slow and can be inefficient for larger proteins – Electroblotting • Used to transfer proteins from gels, is much faster than diffusion, involves electrical current-mediated mobilization of protein through a buffer-saturated transfer stack • Several varieties including Tank (wet), Semi-Dry, and Rapid Semi-Dry 6 Biotechnology Explorer™ | explorer. bio-rad. com
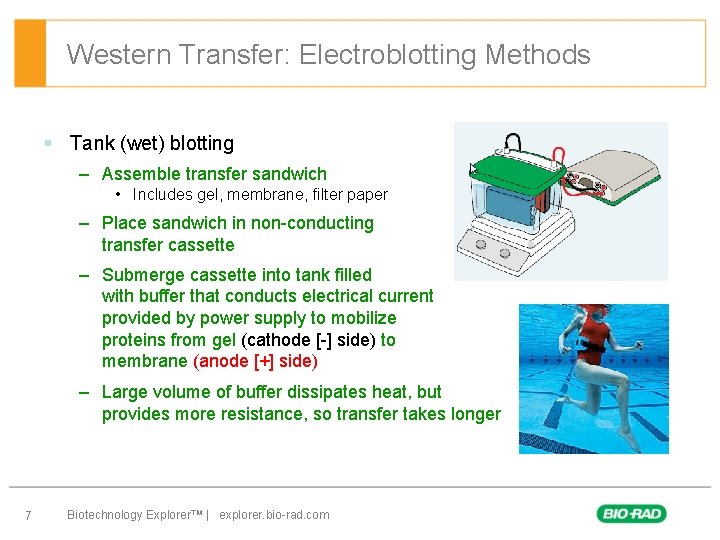
Western Transfer: Electroblotting Methods § Tank (wet) blotting – Assemble transfer sandwich • Includes gel, membrane, filter paper – Place sandwich in non-conducting transfer cassette – Submerge cassette into tank filled with buffer that conducts electrical current provided by power supply to mobilize proteins from gel (cathode [-] side) to membrane (anode [+] side) – Large volume of buffer dissipates heat, but provides more resistance, so transfer takes longer 7 Biotechnology Explorer™ | explorer. bio-rad. com

Western Transfer: Electroblotting Methods § Semi-Dry blotting – Assemble transfer sandwich, which is pre-saturated in transfer buffer – Distance between electrodes is very small (only the width of the transfer sandwich) – Smaller volume of buffer decreases ability to dissipate heat, but also lowers resistance, allowing transfer to occur more rapidly 8 Biotechnology Explorer™ | explorer. bio-rad. com

Western Transfer: Comparison of Electroblotting Methods Tank Blotting Semi-Dry Blotting Traditional 9 Rapid Transfer time 30 min – overnight 15 – 60 min 3 – 15 min Handling convenience Manual assembly of transfer components Prepackaged, presaturated components Transfer parameters Widest range of power settings and transfer times Power and transfer time limited due to lack of cooling options Preinstalled, customizable programs, or userprogrammable settings Temperature control Cooling with ice pack or refrigerated water circulator None Buffer requirement 1 -12 L, system dependent 250 ml per blot No additional buffer required Biotechnology Explorer™ | explorer. bio-rad. com
Trans-Blot Turbo Rapid Semi-Dry Blotting – The Easy-Bake oven of western blotting 10 Biotechnology Explorer™ | explorer. bio-rad. com

Trans-Blot Turbo Rapid Semi-Dry Blotting – Advantages • • • 11 Rapid semi-dry system Preassembled membrane packs Bulk consumables are in the works Individual transfer trays = flexible start times Can transfer up to 4 Mini Gels at a time Biotechnology Explorer™ | explorer. bio-rad. com

TGX Gel Technology – What is TGX? • TGX = Tris Glycine e. Xtended PAGE gels • Modification of traditional Laemmli system – What’s different from traditional SDS-PAGE gels? • Extended shelf life - gels stable for 12 months • Faster run times, because TGX gels can withstand higher voltages • More cost effective than traditional PAGE gels • Available in Stain-Free version 12 Biotechnology Explorer™ | explorer. bio-rad. com
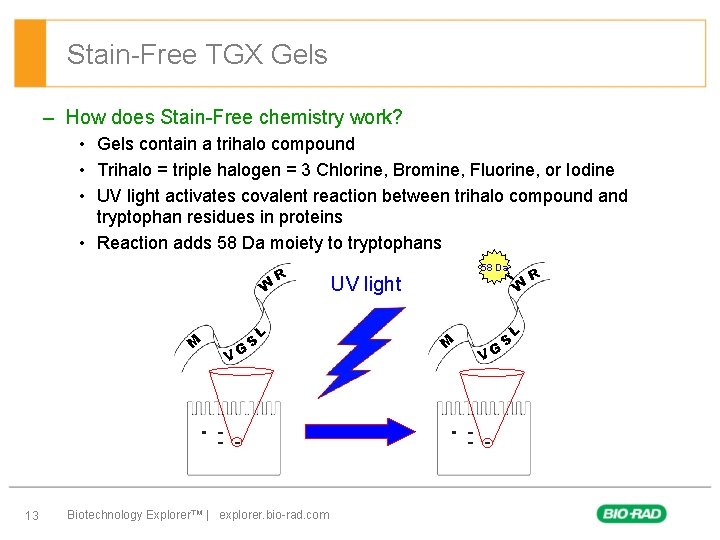
Stain-Free TGX Gels – How does Stain-Free chemistry work? • Gels contain a trihalo compound • Trihalo = triple halogen = 3 Chlorine, Bromine, Fluorine, or Iodine • UV light activates covalent reaction between trihalo compound and tryptophan residues in proteins • Reaction adds 58 Da moiety to tryptophans W M 13 VG S R L Biotechnology Explorer™ | explorer. bio-rad. com 58 Da UV light W M VG S L R
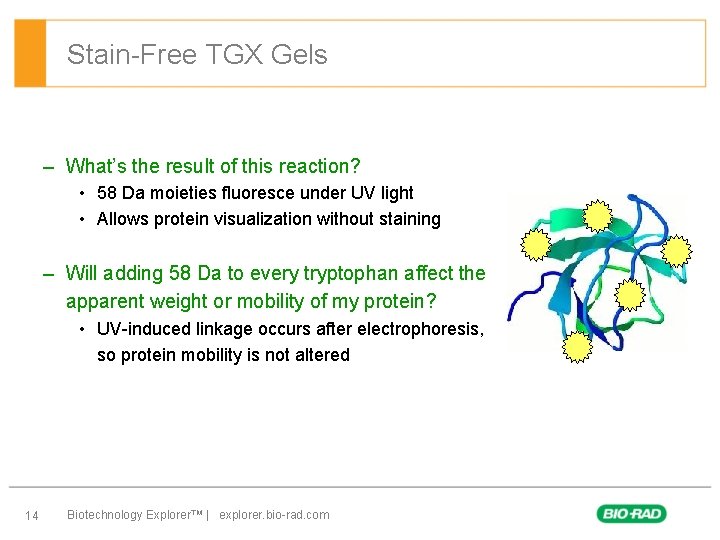
Stain-Free TGX Gels – What’s the result of this reaction? • 58 Da moieties fluoresce under UV light • Allows protein visualization without staining – Will adding 58 Da to every tryptophan affect the apparent weight or mobility of my protein? • UV-induced linkage occurs after electrophoresis, so protein mobility is not altered 14 Biotechnology Explorer™ | explorer. bio-rad. com
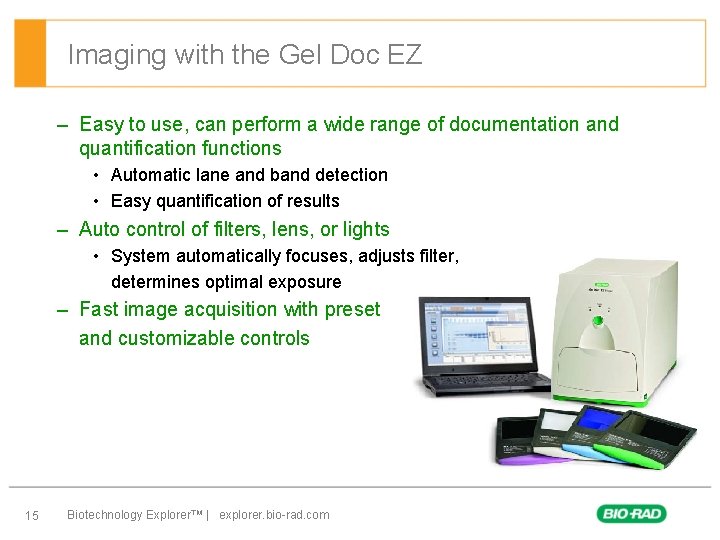
Imaging with the Gel Doc EZ – Easy to use, can perform a wide range of documentation and quantification functions • Automatic lane and band detection • Easy quantification of results – Auto control of filters, lens, or lights • System automatically focuses, adjusts filter, determines optimal exposure – Fast image acquisition with preset and customizable controls 15 Biotechnology Explorer™ | explorer. bio-rad. com
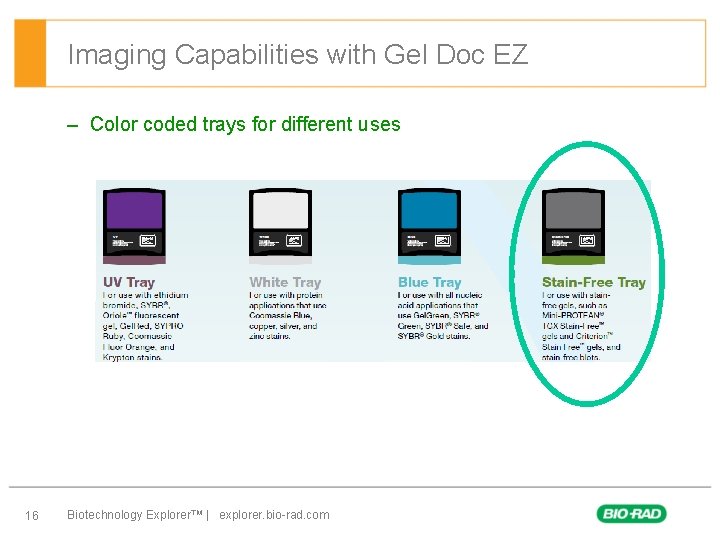
Imaging Capabilities with Gel Doc EZ – Color coded trays for different uses 16 Biotechnology Explorer™ | explorer. bio-rad. com

Rapid V 3 Stain-Free Western Blotting Lab – – – 17 Run samples on Stain-Free TGX gels Visualize protein separation using Gel Doc EZ Transfer proteins to nitrocellulose using Trans-Blot Turbo Verify protein transfer using Gel Doc EZ Immunodetection for myosin light chain Biotechnology Explorer™ | explorer. bio-rad. com

Comparative Proteomics Kit II: Western Blot Module 18 § Applied immunology activity § Use antibodies as detection tools § Laboratory extension to Comparative Proteomics Kit I: Protein Profiler Module § Includes sufficient materials for 8 student workstations § Obtain fish samples, extract protein, visualize proteome after SDS-PAGE, specifically detect myosin light chain Biotechnology Explorer™ | explorer. bio-rad. com
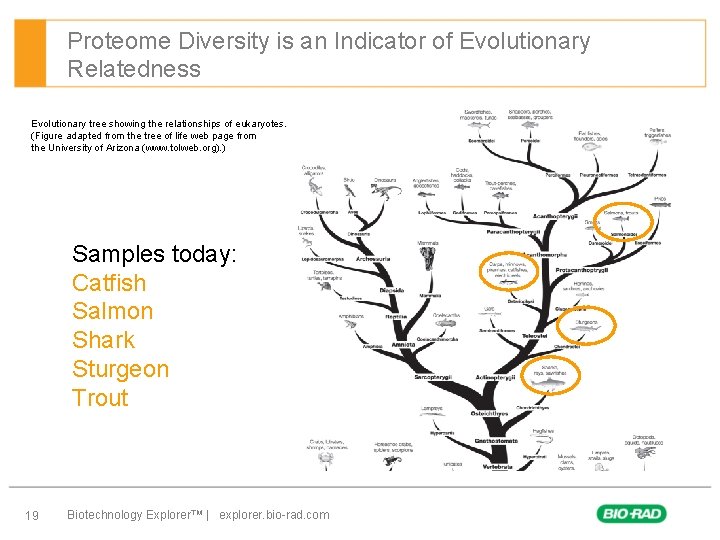
Proteome Diversity is an Indicator of Evolutionary Relatedness Evolutionary tree showing the relationships of eukaryotes. (Figure adapted from the tree of life web page from the University of Arizona (www. tolweb. org). ) Samples today: Catfish Salmon Shark Sturgeon Trout 19 Biotechnology Explorer™ | explorer. bio-rad. com
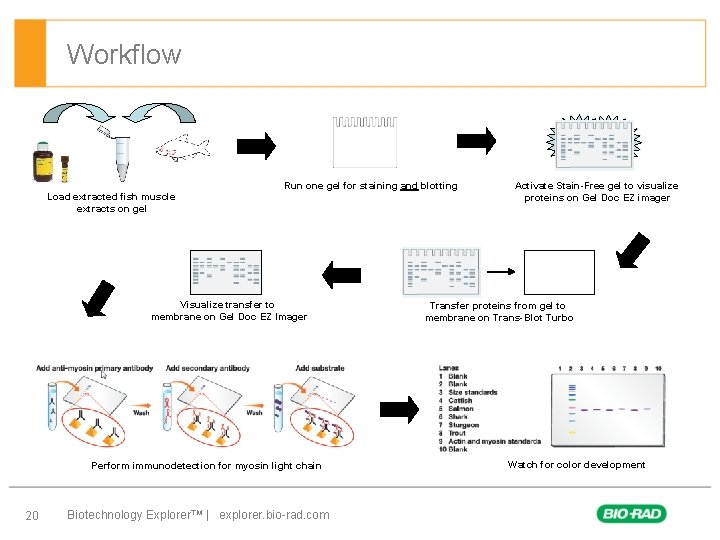
Workflow Run one gel for staining and blotting Load extracted fish muscle extracts on gel Visualize transfer to membrane on Gel Doc EZ Imager Perform immunodetection for myosin light chain 20 Biotechnology Explorer™ | explorer. bio-rad. com Activate Stain-Free gel to visualize proteins on Gel Doc EZ imager Transfer proteins from gel to membrane on Trans-Blot Turbo Watch for color development
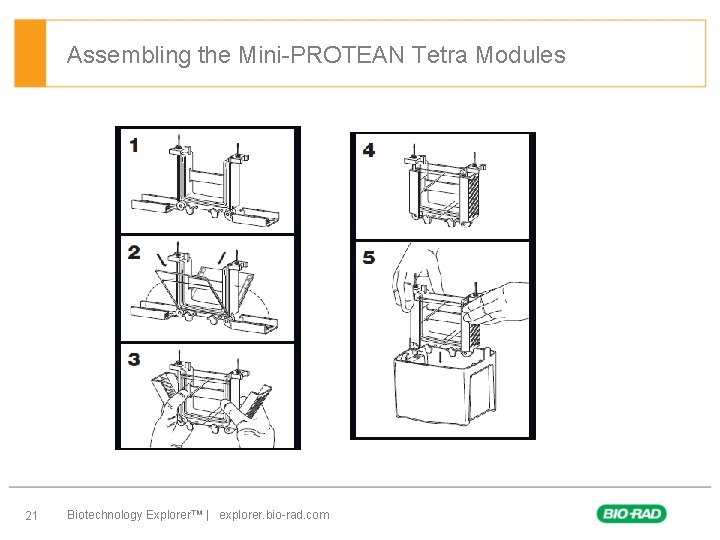
Assembling the Mini-PROTEAN Tetra Modules 21 Biotechnology Explorer™ | explorer. bio-rad. com
Loading and Running the Gels § Samples already heated to 95 o. C in Laemmli buffer § Load 5 ul Kaleidoscope Standard § Load 3 ul fish samples and Actin/Myosin § Run gel 300 V, 18 min 22 Biotechnology Explorer™ | explorer. bio-rad. com
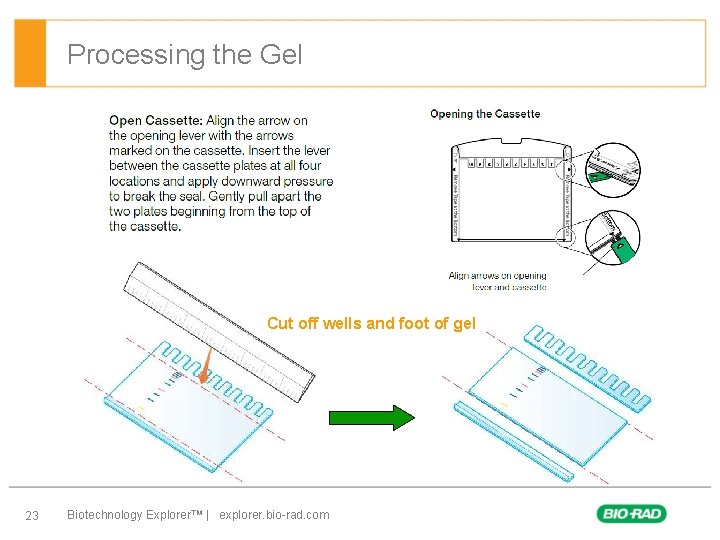
Processing the Gel Cut off wells and foot of gel 23 Biotechnology Explorer™ | explorer. bio-rad. com
Activating Stain-Free TGX Gels Image. Lab 24 Biotechnology Explorer™ | explorer. bio-rad. com
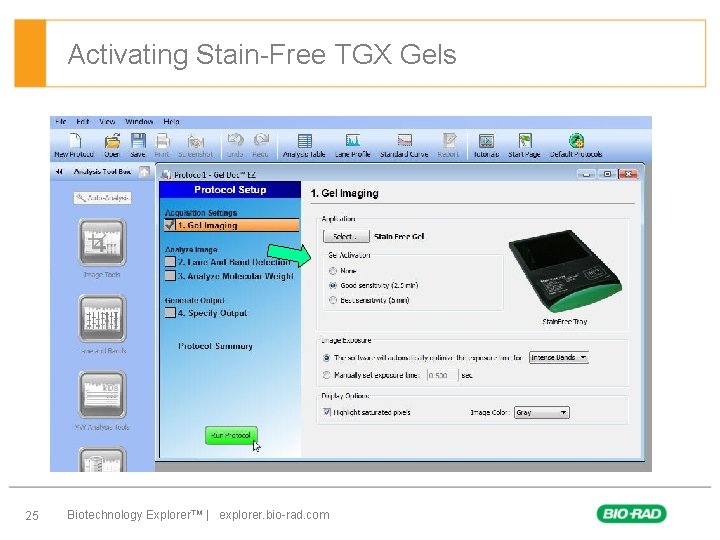
Activating Stain-Free TGX Gels 25 Biotechnology Explorer™ | explorer. bio-rad. com
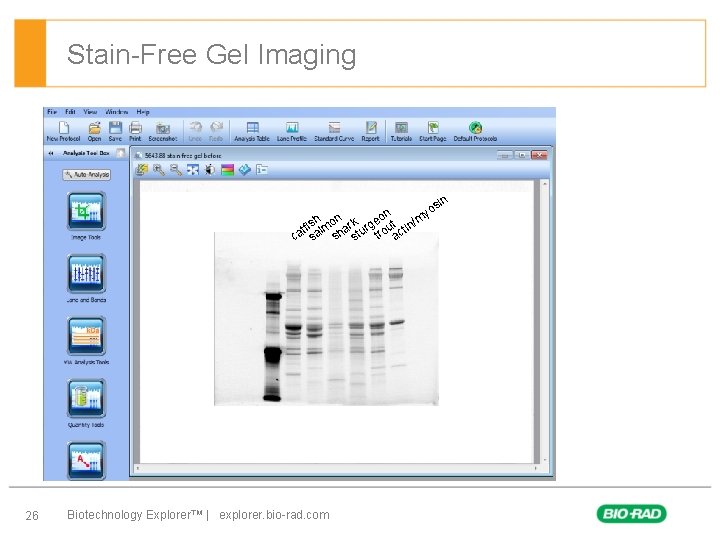
Stain-Free Gel Imaging § First level – Second level • Third level – Forth level 26 in os n y o m h n fis lmo hark urge out ctin/ t a t r a t a s s c s Biotechnology Explorer™ | explorer. bio-rad. com
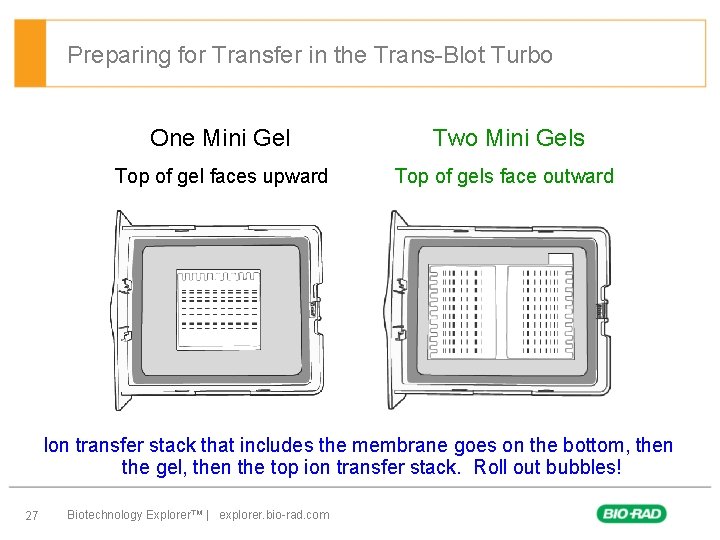
Preparing for Transfer in the Trans-Blot Turbo One Mini Gel Two Mini Gels Top of gel faces upward Top of gels face outward Ion transfer stack that includes the membrane goes on the bottom, then the gel, then the top ion transfer stack. Roll out bubbles! 27 Biotechnology Explorer™ | explorer. bio-rad. com
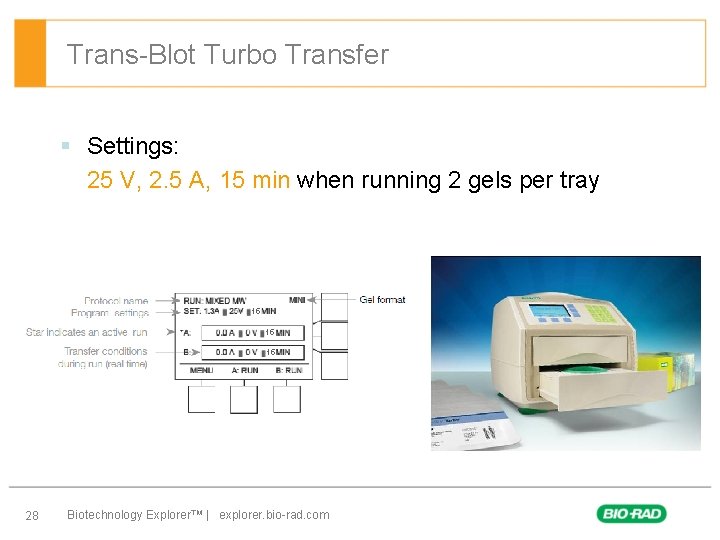
Trans-Blot Turbo Transfer § Settings: 25 V, 2. 5 A, 15 min when running 2 gels per tray 15 15 15 28 Biotechnology Explorer™ | explorer. bio-rad. com
Stain-Free Blot Imaging § First level – Second level • Third level – Forth level 29 Biotechnology Explorer™ | explorer. bio-rad. com
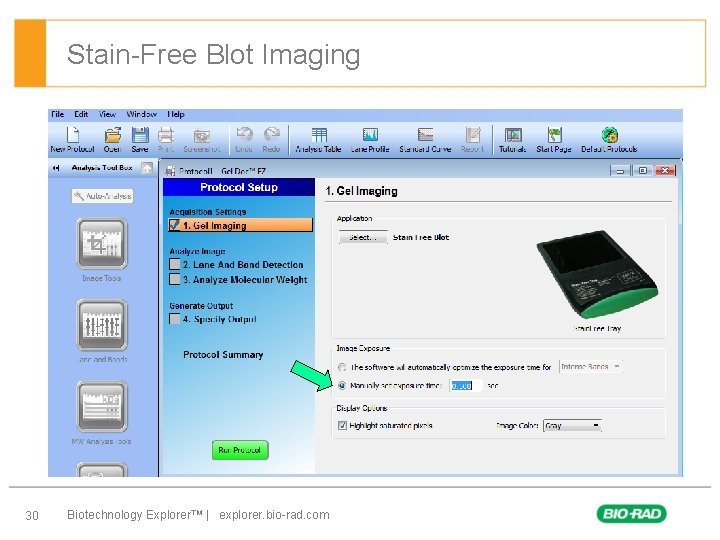
Stain-Free Blot Imaging § First level – Second level • Third level – Forth level 30 Biotechnology Explorer™ | explorer. bio-rad. com

Stain-Free Blot Imaging § First level – Second level • Third level – Forth level 31 sin o n y m eo h n fis lmo hark turg out ctin/ t a r a t s s a c s Biotechnology Explorer™ | explorer. bio-rad. com
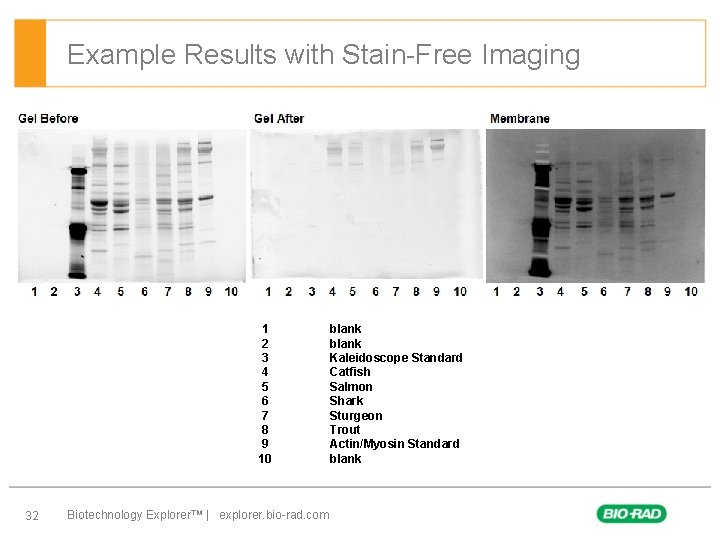
Example Results with Stain-Free Imaging 1 2 3 4 5 6 7 8 9 10 32 Biotechnology Explorer™ | explorer. bio-rad. com blank Kaleidoscope Standard Catfish Salmon Shark Sturgeon Trout Actin/Myosin Standard blank

Western Blotting: Blocking Buffer Remove membrane from the blotting sandwich and immerse in 25 ml of blocking solution for 10 minutes 5% non-fat milk: Prevents the primary antibody from binding randomly to the membrane Phosphate buffered saline (PBS): Provides the correct environment (p. H, Salt) to maintain protein shape 0. 025% Tween 20: non-ionic detergent that prevents non-specific binding of antibodies to the membrane 33 Biotechnology Explorer™ | explorer. bio-rad. com
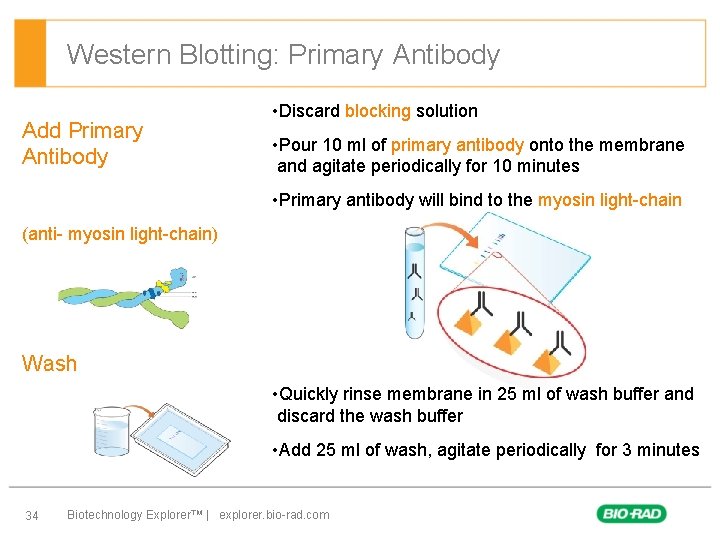
Western Blotting: Primary Antibody Add Primary Antibody • Discard blocking solution • Pour 10 ml of primary antibody onto the membrane and agitate periodically for 10 minutes • Primary antibody will bind to the myosin light-chain (anti- myosin light-chain) Wash • Quickly rinse membrane in 25 ml of wash buffer and discard the wash buffer • Add 25 ml of wash, agitate periodically for 3 minutes 34 Biotechnology Explorer™ | explorer. bio-rad. com
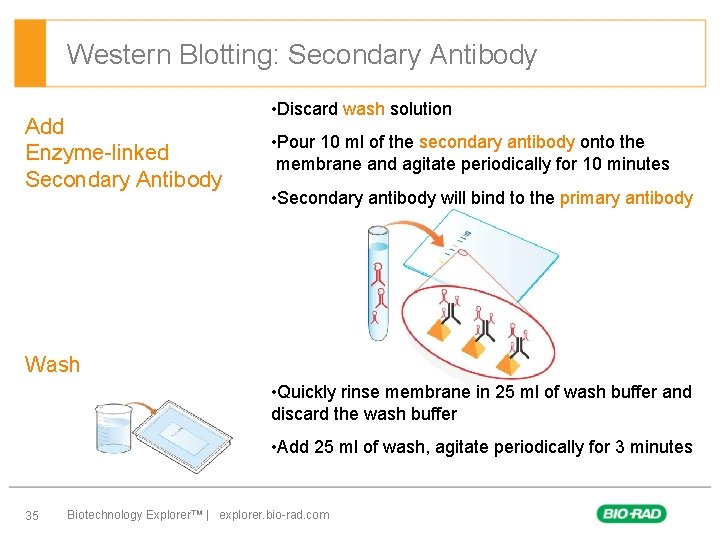
Western Blotting: Secondary Antibody Add Enzyme-linked Secondary Antibody • Discard wash solution • Pour 10 ml of the secondary antibody onto the membrane and agitate periodically for 10 minutes • Secondary antibody will bind to the primary antibody Wash • Quickly rinse membrane in 25 ml of wash buffer and discard the wash buffer • Add 25 ml of wash, agitate periodically for 3 minutes 35 Biotechnology Explorer™ | explorer. bio-rad. com
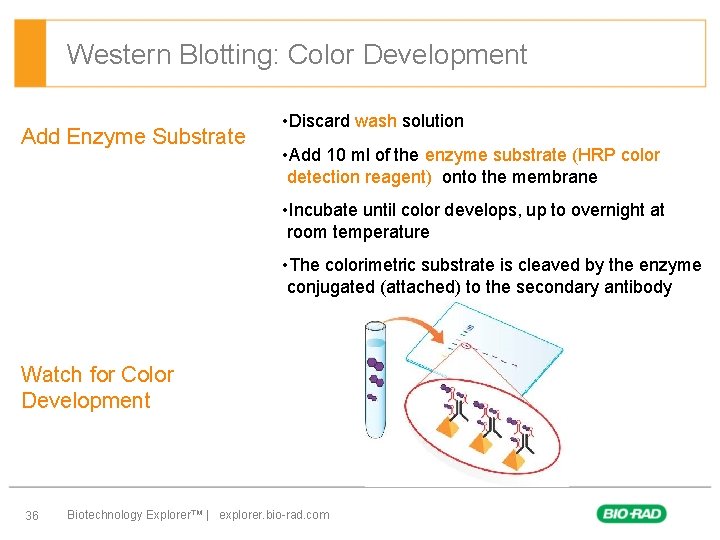
Western Blotting: Color Development Add Enzyme Substrate • Discard wash solution • Add 10 ml of the enzyme substrate (HRP color detection reagent) onto the membrane • Incubate until color develops, up to overnight at room temperature • The colorimetric substrate is cleaved by the enzyme conjugated (attached) to the secondary antibody Watch for Color Development 36 Biotechnology Explorer™ | explorer. bio-rad. com
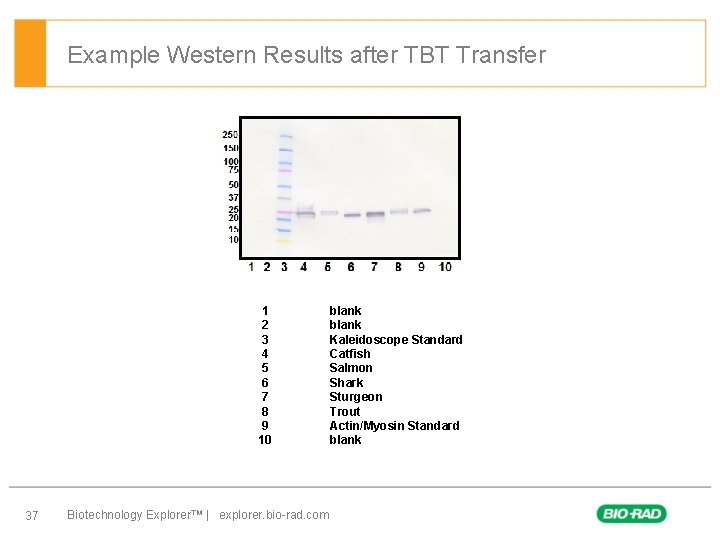
Example Western Results after TBT Transfer 1 2 3 4 5 6 7 8 9 10 37 Biotechnology Explorer™ | explorer. bio-rad. com blank Kaleidoscope Standard Catfish Salmon Shark Sturgeon Trout Actin/Myosin Standard blank
Western Blot Storage Store Membrane • Rinse the developed membrane twice with distilled water and blot dry • Air dry for 30 min-1 hr and store in lab notebook 38 Biotechnology Explorer™ | explorer. bio-rad. com
Like what you see? Find out more! § Visit us on the web – www. explorer. bio-rad. com § Rapid Western + V 3 Stain-Free Workflow Application Note coming soon! § Bio-Rad Curriculum and Training Specialist – Damon Tighe • Damon_Tighe@bio-rad. com 39 Biotechnology Explorer™ | explorer. bio-rad. com
Click to add title here § First level – Second level • Third level – Forth level 40 Biotechnology Explorer™ | explorer. bio-rad. com
- Slides: 40