Rapid Cycle Analysis for Early Detection of Vaccine












- Slides: 23

Rapid Cycle Analysis for Early Detection of Vaccine Adverse Events Richard Platt, MD, MSc for the CDC Vaccine Safety Datalink Investigators Harvard Pilgrim Health Care and Harvard Medical School 1

Why Do We Need Early Detection Systems? • Rare adverse events may be impossible to detect in pre-licensure studies • Spontaneous reports to passive surveillance systems, e. g. VAERS, often need rapid follow-up • Designing follow-up studies can take months to years using traditional approaches 2

Rapid Cycle Analysis • A modern approach to surveillance that takes advantage of VSD’s strengths • Update data on all vaccines and all subsequent outcomes every week • Choose vaccines and potential adverse events to monitor • Conduct weekly analysis 3

Sequential Analysis Methods • Early data contribute to every subsequent analysis • Repeated statistical testing of the same data requires special methods • New method: Maximized SPRT (Kulldorff et al. , 2004) • A refinement of sequential probability ratio testing (Wald, 1955) 4

Example: Rotavirus vaccine and intussusception 1999 Vaccine licensed Aug 98 15 VAERs reports through Jul 99 Vaccine suspended Withdrawn 5

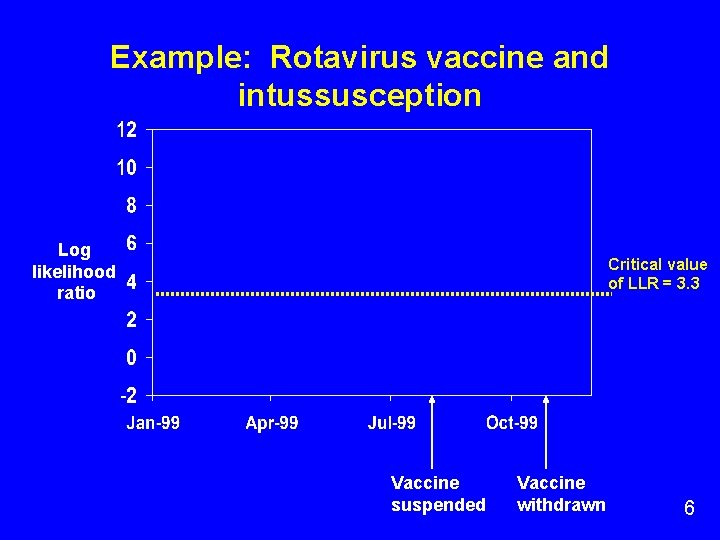
Example: Rotavirus vaccine and intussusception Log likelihood ratio Critical value of LLR = 3. 3 Vaccine suspended Vaccine withdrawn 6

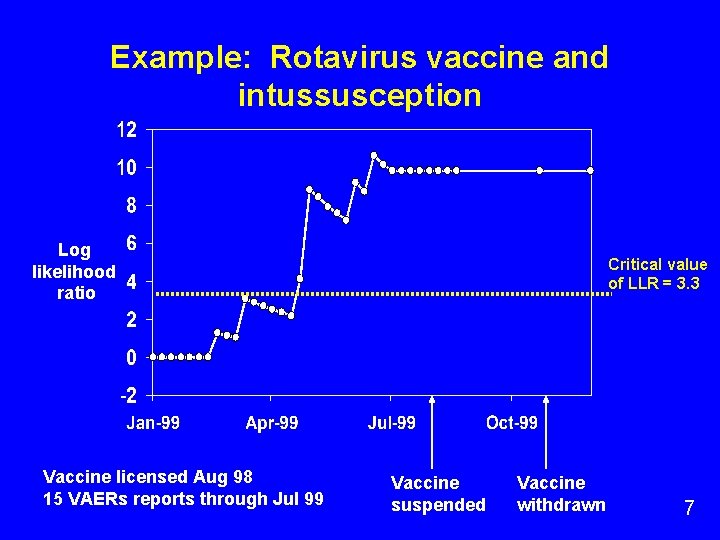
Example: Rotavirus vaccine and intussusception Log likelihood ratio Vaccine licensed Aug 98 15 VAERs reports through Jul 99 Critical value of LLR = 3. 3 Vaccine suspended Vaccine withdrawn 7

Rapid Cycle Analysis – Ongoing Surveillance via VSD • Meningococcal conjugate vaccine and Guillain-Barre syndrome • Rotavirus vaccine and intussusception, gastrointestinal bleeding, and other events • HPV, Tdap, MMRV, influenza vaccines – being implemented 8

Implementing Rapid Cycle Analysis • For each vaccine, choose the outcomes of interest • Choose the comparison method – concurrent controls, historical rates, or both • Create programs to generate aggregate data from the 8 VSD sites • Program analysis and run weekly 9

Choosing Outcomes Select outcomes that are: • Clearly defined – e. g. , Guillain-Barre syndrome or seizures rather than “neurologic problems” • Acute-onset • Relatively uncommon • Plausible 10

Concurrent Comparison Analysis • Uses matched controls, e. g. , patients making preventive visits – Advantage: Avoids false signaling or missed signals due to secular trends – Limitations: • Need to define appropriate control groups – not simple! • Vaccines may be adopted rapidly, leaving few controls 11

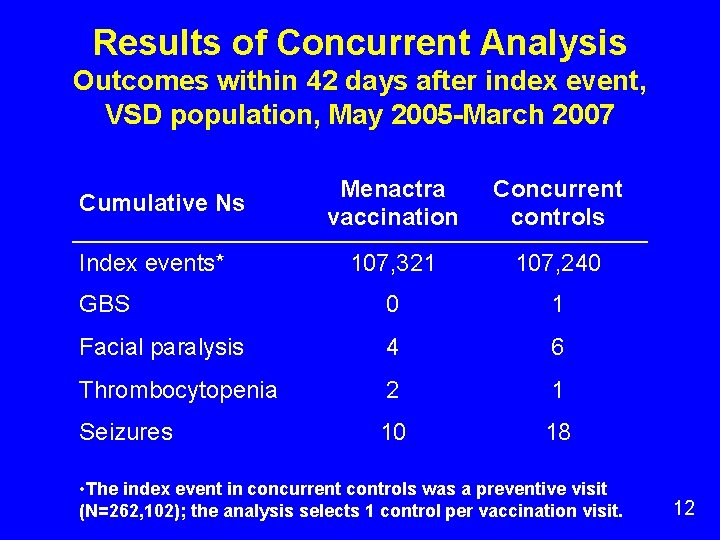
Results of Concurrent Analysis Outcomes within 42 days after index event, VSD population, May 2005 -March 2007 Menactra vaccination Concurrent controls 107, 321 107, 240 GBS 0 1 Facial paralysis 4 6 Thrombocytopenia 2 1 Seizures 10 18 Cumulative Ns Index events* • The index event in concurrent controls was a preventive visit (N=262, 102); the analysis selects 1 control per vaccination visit. 12

Historical Comparison Analysis • Uses incidence rates from existing data – Advantage: Knowing the historical rate of rare events allows earlier recognition that a small number of cases among vaccine recipients is unusual – Example: 4 cases of Guillain-Barre syndrome occur in vaccinees, 0 in controls – Limitation: Secular trends 13

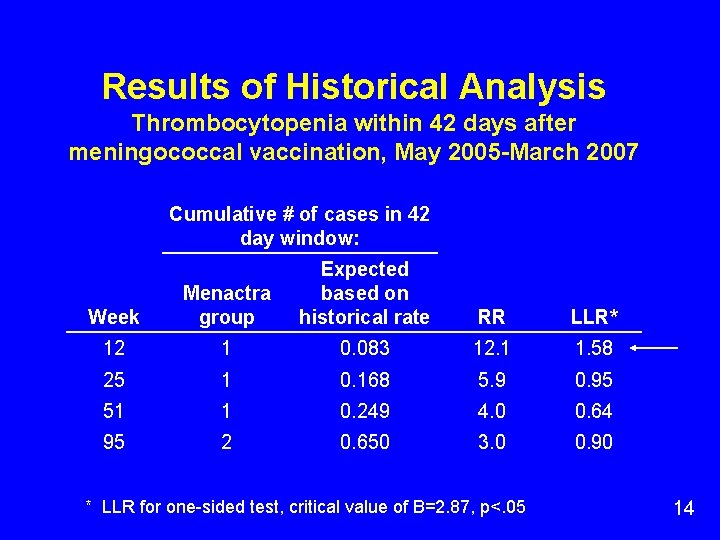
Results of Historical Analysis Thrombocytopenia within 42 days after meningococcal vaccination, May 2005 -March 2007 Cumulative # of cases in 42 day window: Week Menactra group Expected based on historical rate RR LLR* 12 1 0. 083 12. 1 1. 58 25 1 0. 168 5. 9 0. 95 51 1 0. 249 4. 0 0. 64 95 2 0. 650 3. 0 0. 90 * LLR for one-sided test, critical value of B=2. 87, p<. 05 14

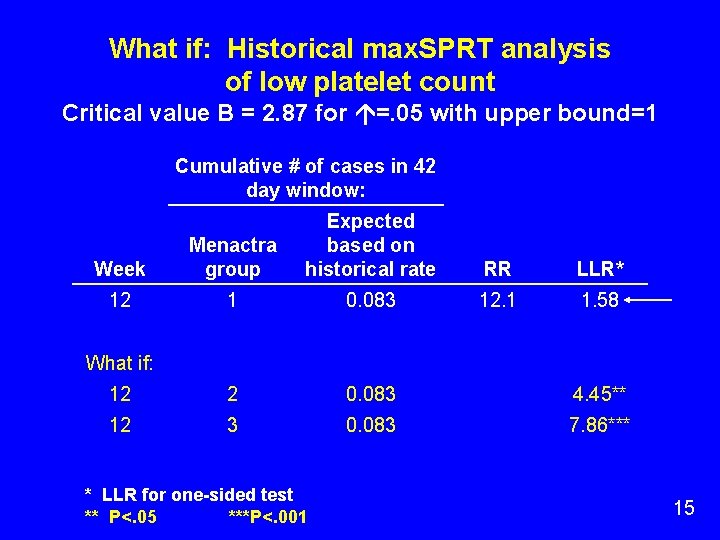
What if: Historical max. SPRT analysis of low platelet count Critical value B = 2. 87 for =. 05 with upper bound=1 Cumulative # of cases in 42 day window: Week Menactra group Expected based on historical rate RR LLR* 12 1 0. 083 12. 1 1. 58 12 2 0. 083 4. 45** 12 3 0. 083 7. 86*** What if: * LLR for one-sided test ** P<. 05 ***P<. 001 15

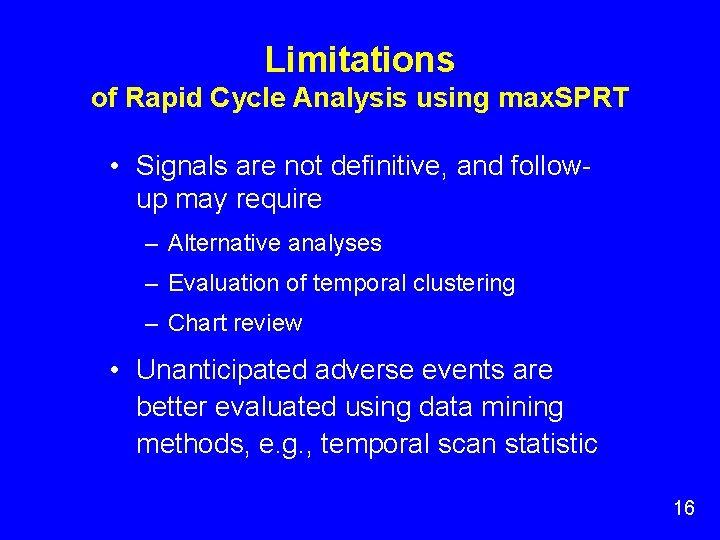
Limitations of Rapid Cycle Analysis using max. SPRT • Signals are not definitive, and followup may require – Alternative analyses – Evaluation of temporal clustering – Chart review • Unanticipated adverse events are better evaluated using data mining methods, e. g. , temporal scan statistic 16

Next Steps • VSD plans to implement surveillance rapidly whenever a new vaccine is introduced • Refine methods of analysis for new situations, e. g. zoster • Add new populations 17

Coming soon: CERTs Health Plan Consortium for Public Health • Goal: Improve the safety and safe use of marketed vaccines and prescription drugs by studying their use in health plan members • Target population: 100 million • A planned activity of the Centers for Education and Research on Therapeutics (CERTs) – Created under Congressional mandate to be a trusted national resource in therapeutics – Administered by AHRQ in consultation with FDA – Accepted processes for administering public-private partnerships 18

CERTs Health Plan Consortium for Public Health – Aims • Timely risk identification and quantification – Prospective evaluation of new therapeutics captured by health plan data • focus on pre-defined list of potential problems – Detailed followup of selected problems • Identification of potentially unsafe use of preventive and therapeutic agents • Other topics, subject to Board approval 19

CERTs Health Plan Consortium for Public Health • Structure: Public-private partnership – health plans, federal agencies, industry, professional societies, public, foundations, academic community • Data: health plans’ automated data (claims+) with access to full text medical records • Transparency: – Proposed protocols available in advance for public comment – Final protocols publicly available – Final results publicly available • Confidentiality and privacy: – Individuals: HIPAA/IRB compliant – Health plans: identity and proprietary data protected 20

CERTs Health Plan Consortium for Public Health – Funding • Infrastructure requires core funding • Individual projects will require separate funding 21

CERTs Health Plan Consortium for Public Health • Existing health plan data allow substantial enhancement of timeliness, power, and efficiency of post-marketing studies of therapeutics • This information can/should complement other sources – Medicare/Medicaid, VA, Vaccine Safety Datalink 22

Collaborators – partial list • • James Baggs, CDC Jeff Brown, Harvard Arnold Chan, CERT Bob Davis, CDC Inna Dashevsky, CERT Rich Fox, Harvard David Graham, FDA Margarette Kolczak, CDC • Martin Kulldorff, Harvard • • • Ned Lewis, Kaiser Kim Lane, CERT Renny Li, Harvard Tracy Lieu, Harvard Parker Pettus, CERT Irene Shui, Harvard Eric Weintraub, CDC Katherine Yih, Harvard Ruihua Yin, Harvard Health plan-based VSD and CERT teams 23