RANOLAZINE Dr Merajuddin shah MD DM Cardiology AlKareem





- Slides: 31

RANOLAZINE Dr. Merajuddin shah, MD, DM (Cardiology) Al-Kareem Cardiac Center, Srinagar, Kashmir

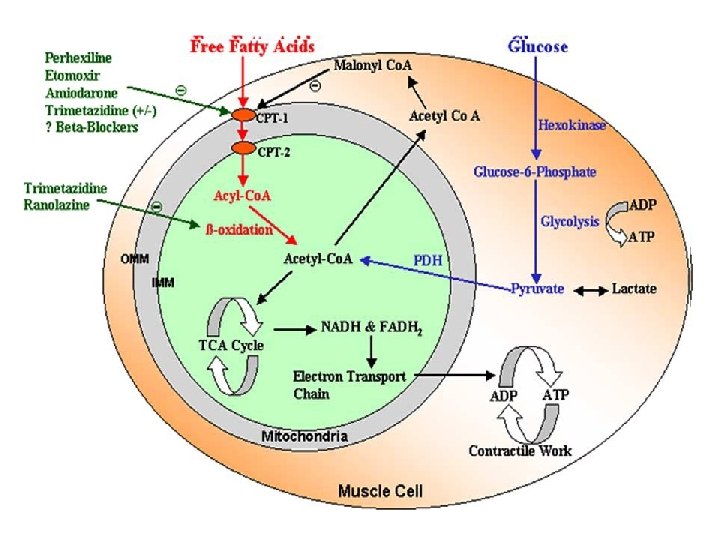
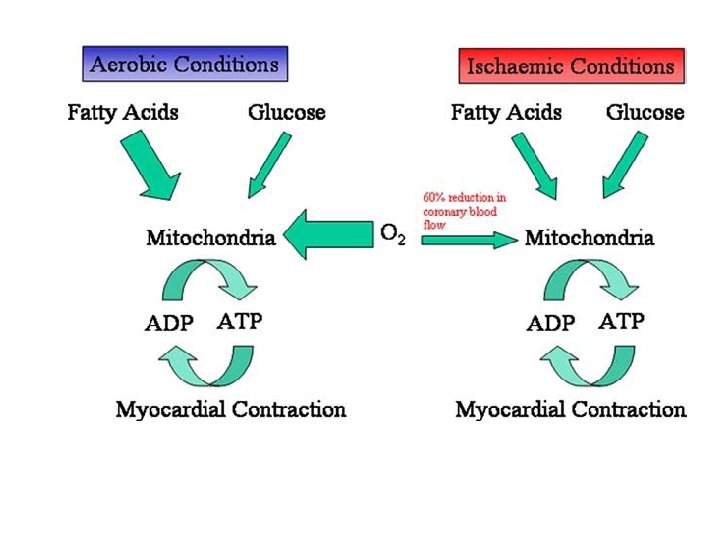
METABOLIC MANUPULATION OF ISCHEMIC HEART DISEASE. A NOVEL APPROACH TO TREATMENT ----Leong Lee , EHJ, 2004

RANOLAZINE A Piperazine Derivative


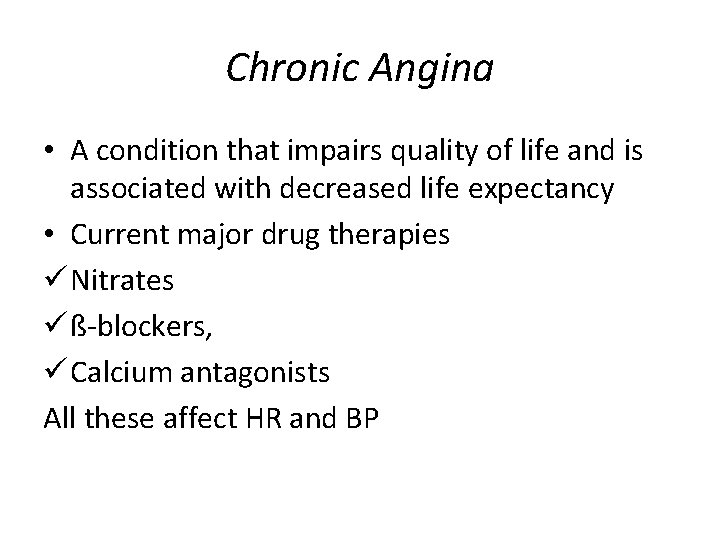
Chronic Angina • A condition that impairs quality of life and is associated with decreased life expectancy • Current major drug therapies ü Nitrates ü ß-blockers, ü Calcium antagonists All these affect HR and BP

Ranolazine • A drug that reduces angina symptoms, with a mechanism of action different from that of currently available pharmacological therapies. • Do not affect HR & BP. Ranolazine was approved on January 27, 2006, in the United States for use in patients with chronic angina who continue to be symptomatic on ß-blockers, calcium antagonists, or nitrates.

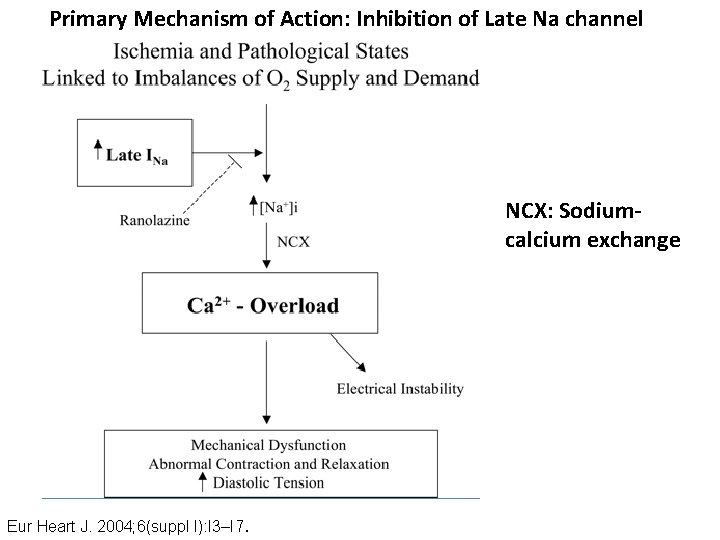
Primary Mechanism of Action: Inhibition of Late Na channel NCX: Sodiumcalcium exchange Eur Heart J. 2004; 6(suppl I): I 3–I 7.

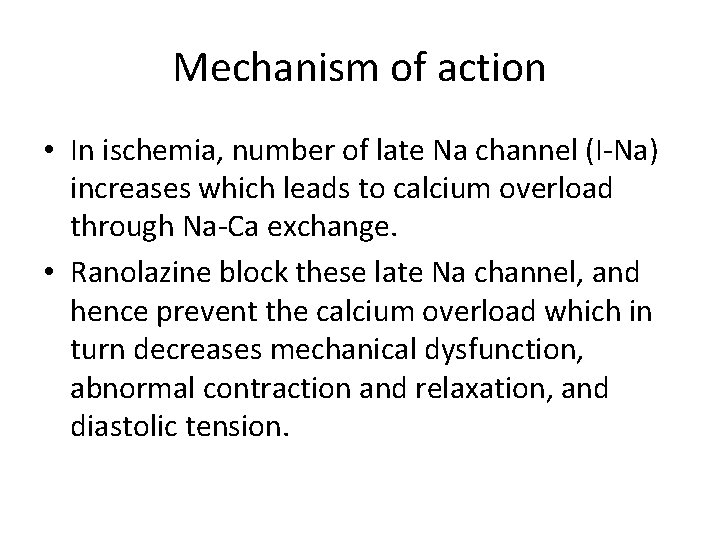
Mechanism of action • In ischemia, number of late Na channel (I-Na) increases which leads to calcium overload through Na-Ca exchange. • Ranolazine block these late Na channel, and hence prevent the calcium overload which in turn decreases mechanical dysfunction, abnormal contraction and relaxation, and diastolic tension.

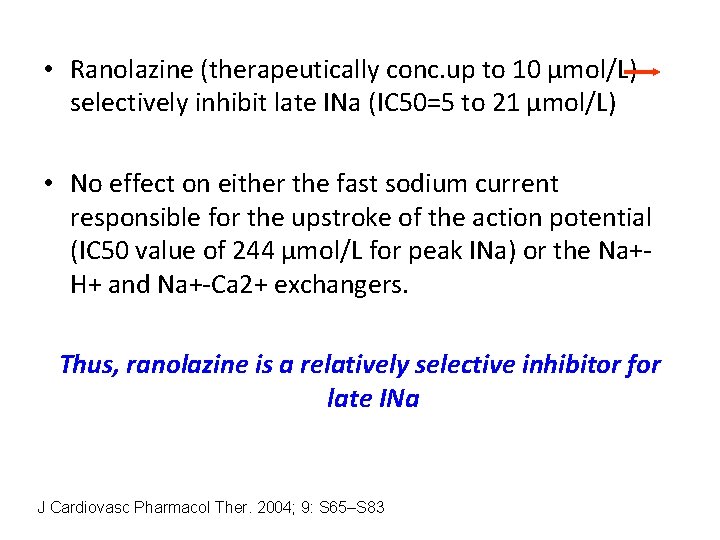
• Ranolazine (therapeutically conc. up to 10 µmol/L) selectively inhibit late INa (IC 50=5 to 21 µmol/L) • No effect on either the fast sodium current responsible for the upstroke of the action potential (IC 50 value of 244 µmol/L for peak INa) or the Na+H+ and Na+-Ca 2+ exchangers. Thus, ranolazine is a relatively selective inhibitor for late INa J Cardiovasc Pharmacol Ther. 2004; 9: S 65–S 83

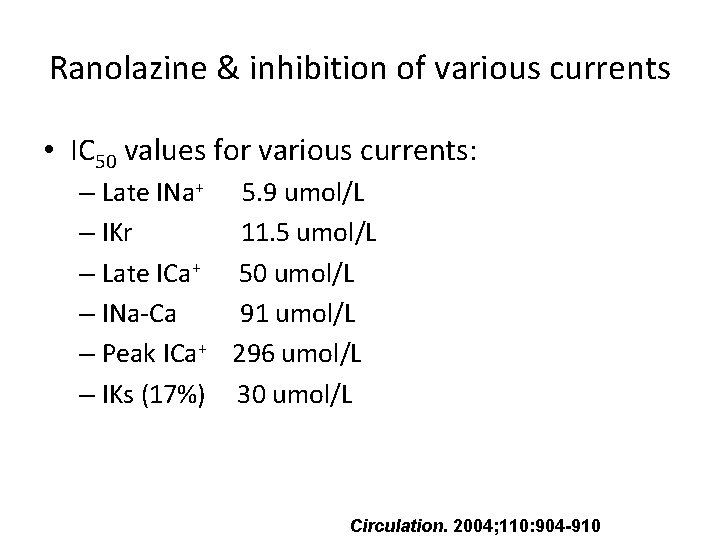
Ranolazine & inhibition of various currents • IC 50 values for various currents: – Late INa+ 5. 9 umol/L – IKr 11. 5 umol/L – Late ICa+ 50 umol/L – INa-Ca 91 umol/L – Peak ICa+ 296 umol/L – IKs (17%) 30 umol/L Circulation. 2004; 110: 904 -910

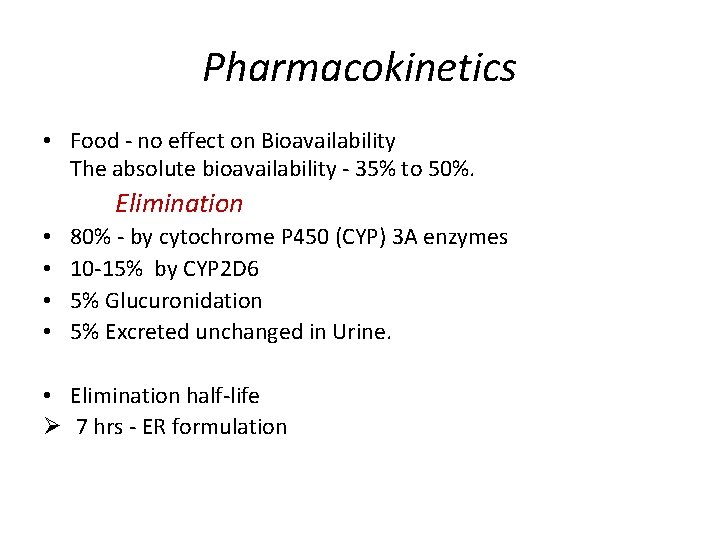
Pharmacokinetics • Food - no effect on Bioavailability The absolute bioavailability - 35% to 50%. Elimination • • 80% - by cytochrome P 450 (CYP) 3 A enzymes 10 -15% by CYP 2 D 6 5% Glucuronidation 5% Excreted unchanged in Urine. • Elimination half-life Ø 7 hrs - ER formulation

Drug–Drug Interaction • Diltiazem (≥ 240 mg daily) - ↑ ranolazine plasma levels - 1. 5 -fold • Ranolazine has no significant effect on diltiazem pharmacokinetics • Verapamil (≥ 360 mg daily) - 2. 3 -fold ↑ in ranolazine plasma levels • Ranolazine increases digoxin concentrations 1. 4 - to 1. 6 -fold at trough &2 -fold at peak plasma levels • Ranolazine is contraindicated in patients on potent and moderately potent CYP 3 A inhibitors such as ketoconazole, diltiazem, verapamil, macrolide antibiotics, HIV protease inhibitors, and grapefruit juice

Drug–Drug Interaction • Simvastatin Cmax is ↑ by 2 -fold after ranolazine; • Simvastatin - no significant effect on ranolazine pharmacokinetics. • In phase II studies of ranolazine with patients on statin drugs, significant increases in creatine kinase, clinical myositis, or elevated liver function tests have not been reported. • No interactions with warfarin • Antiarrhythmic drugs Ø Ø Ø Class Ia: quinidine Class III: dofetilide, sotalol Certain antipsychotics: Thioridazine, ziprasidone

Monotherapy Assessment of Ranolazine In Stable Angina MARISA • Patients withdrawn from other anti-anginals (N = 191 randomized) • Randomized, double-blind, 4 -period crossover – 1 -wk treatment periods – Placebo vs 500, 1000, and 1500 mg bid • Exercise tests after each week of treatment – At trough (12 hr after dosing) – At peak (4 hr after dosing) J Am Coll Cardiol 2004; 43: 1375 -82.

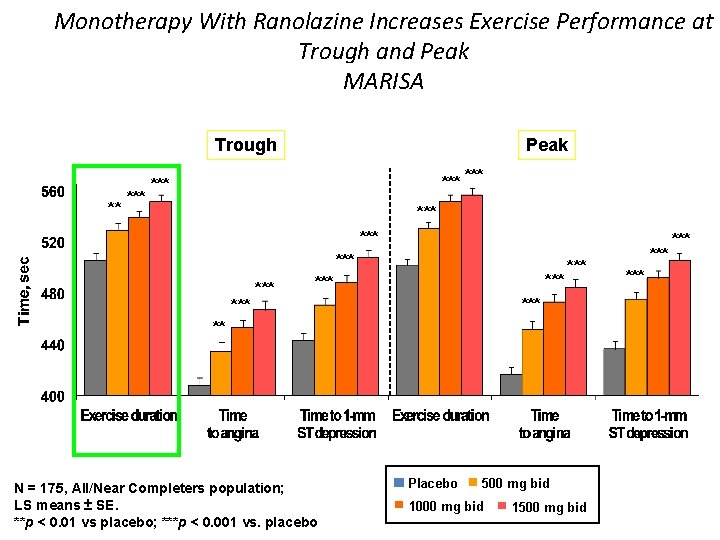
Monotherapy With Ranolazine Increases Exercise Performance at Trough and Peak MARISA Peak Trough ** *** *** *** ** N = 175, All/Near Completers population; LS means ± SE. **p < 0. 01 vs placebo; ***p < 0. 001 vs. placebo Placebo 500 mg bid 1000 mg bid 1500 mg bid *** ***

Combination Assessment of Ranolazine In Stable Angina CARISA • Randomization criteria identical to MARISA except for background therapy – Atenolol 50 mg qd (n = 354), or – Amlodipine 5 mg qd (n = 256), or – Diltiazem CD 180 mg qd (n = 213) • Three parallel groups for 12 wk of treatment – Placebo – Ranolazine 750 mg bid – Ranolazine 1000 mg bid • Exercise testing – At trough after 2, 6, and 12 wk of treatment – At peak after 2 and 12 wk of treatment JAMA 2004; 291: 309 -316.

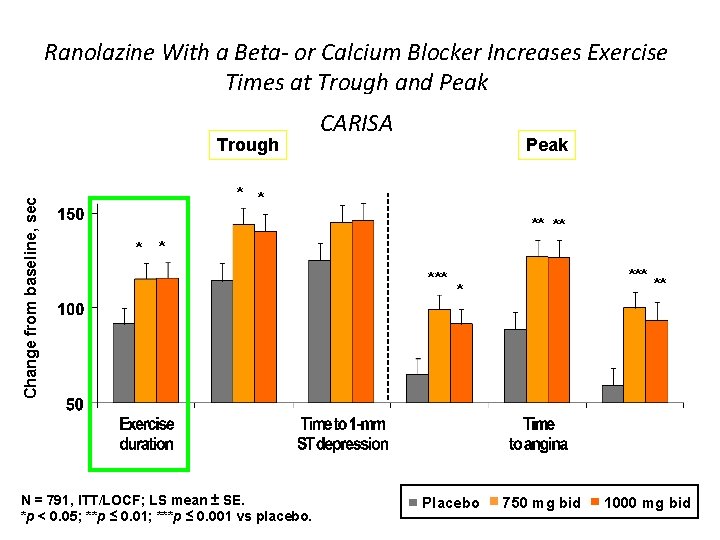
Ranolazine With a Beta- or Calcium Blocker Increases Exercise Times at Trough and Peak Change from baseline, sec Trough CARISA Peak * * ** ** * * N = 791, ITT/LOCF; LS mean ± SE. *p < 0. 05; **p ≤ 0. 01; ***p ≤ 0. 001 vs placebo. *** * Placebo 750 mg bid ** 1000 mg bid

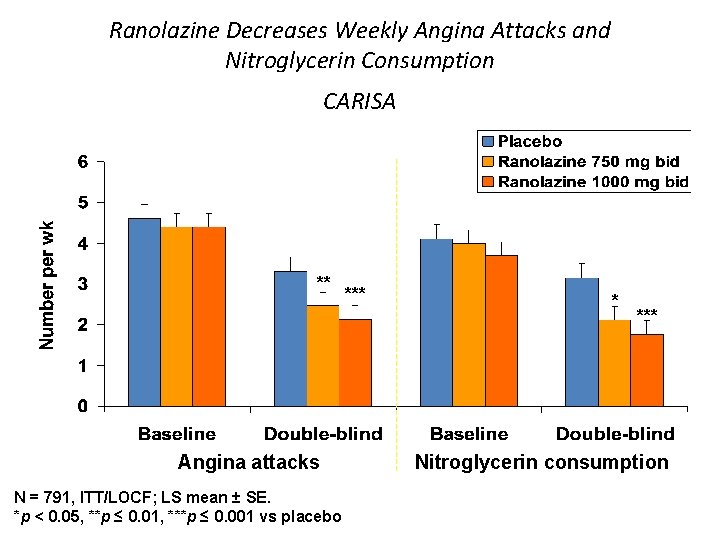
Ranolazine Decreases Weekly Angina Attacks and Nitroglycerin Consumption CARISA ** Angina attacks N = 791, ITT/LOCF; LS mean ± SE. *p < 0. 05, **p ≤ 0. 01, ***p ≤ 0. 001 vs placebo *** * *** Nitroglycerin consumption

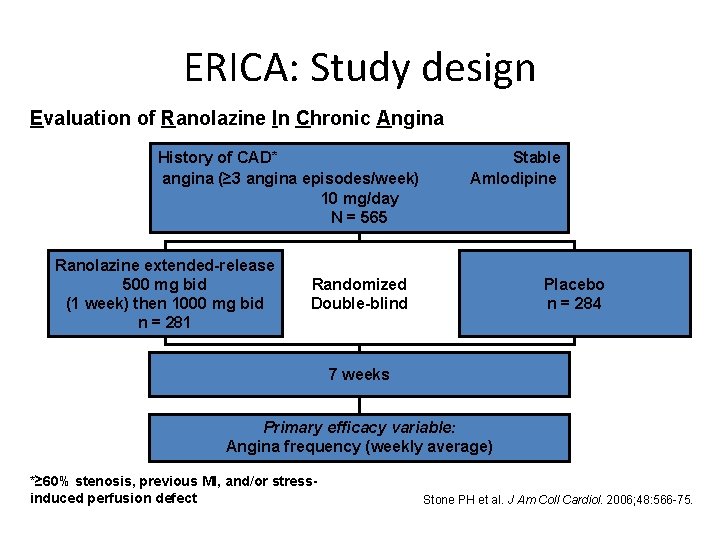
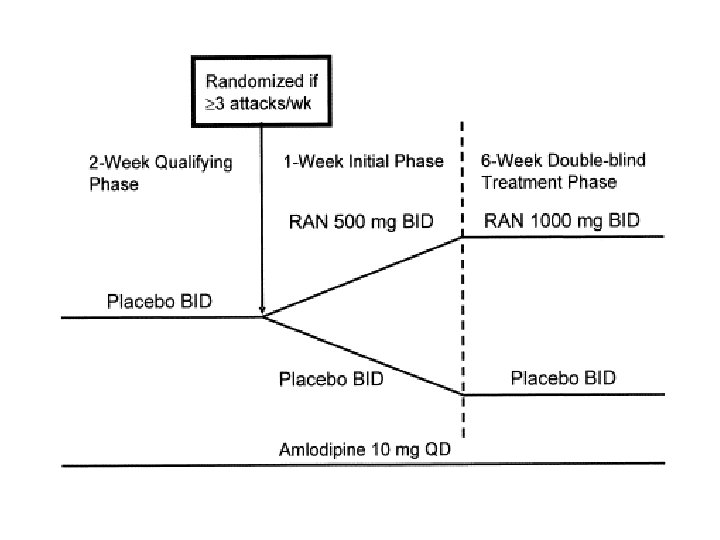
ERICA: Study design Evaluation of Ranolazine In Chronic Angina History of CAD* angina (≥ 3 angina episodes/week) 10 mg/day N = 565 Ranolazine extended-release 500 mg bid (1 week) then 1000 mg bid n = 281 Stable Amlodipine Randomized Double-blind Placebo n = 284 7 weeks Primary efficacy variable: Angina frequency (weekly average) *≥ 60% stenosis, previous MI, and/or stressinduced perfusion defect Stone PH et al. J Am Coll Cardiol. 2006; 48: 566 -75.


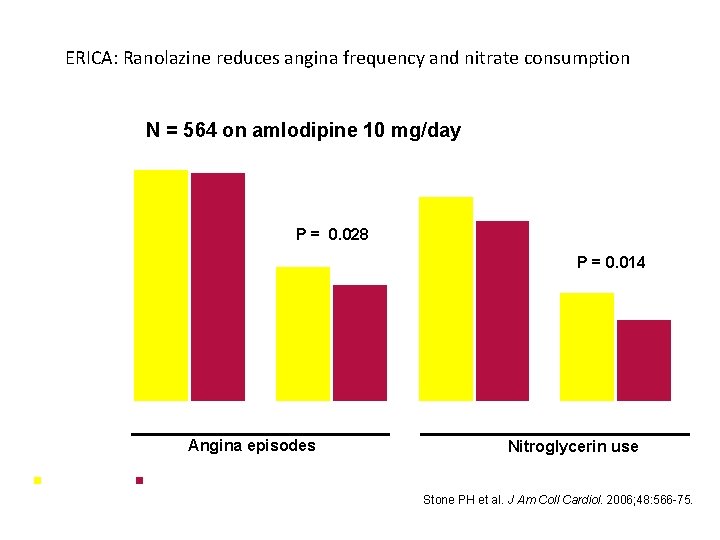
ERICA: Ranolazine reduces angina frequency and nitrate consumption N = 564 on amlodipine 10 mg/day 6 5 P = 0. 028 4 Mean number per week 3 P = 0. 014 2 1 0 Baseline Week 7 Angina episodes Baseline Week 7 Nitroglycerin use Placeboebo RRannanolazine 1000 mg bid Stone PH et al. J Am Coll Cardiol. 2006; 48: 566 -75.

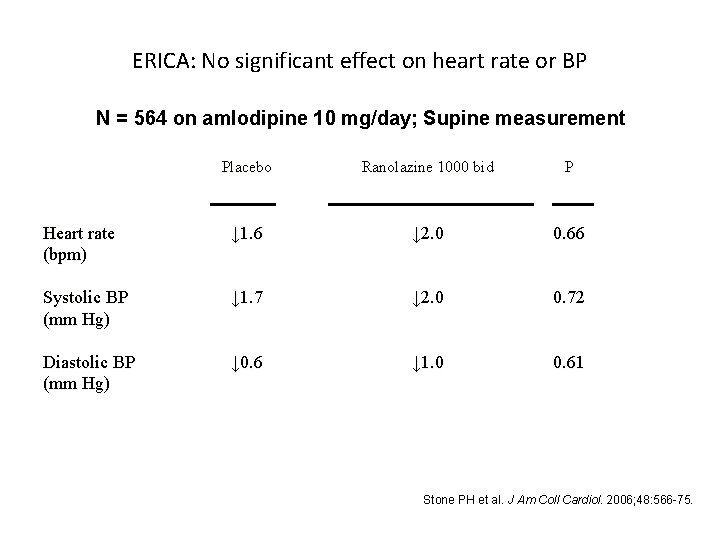
ERICA: No significant effect on heart rate or BP N = 564 on amlodipine 10 mg/day; Supine measurement Placebo Ranolazine 1000 bid P Heart rate (bpm) ↓ 1. 6 ↓ 2. 0 0. 66 Systolic BP (mm Hg) ↓ 1. 7 ↓ 2. 0 0. 72 Diastolic BP (mm Hg) ↓ 0. 6 ↓ 1. 0 0. 61 Stone PH et al. J Am Coll Cardiol. 2006; 48: 566 -75.

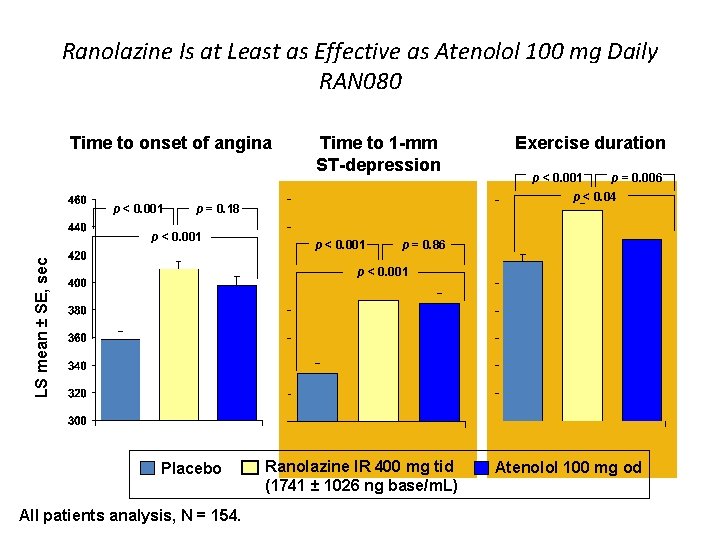
Ranolazine Is at Least as Effective as Atenolol 100 mg Daily RAN 080 Time to onset of angina p < 0. 001 Time to 1 -mm ST-depression LS mean ± SE, sec p < 0. 001 p = 0. 006 p < 0. 04 p = 0. 18 p < 0. 001 Exercise duration p < 0. 001 p = 0. 86 p < 0. 001 Placebo All patients analysis, N = 154. Ranolazine IR 400 mg tid (1741 ± 1026 ng base/m. L) Atenolol 100 mg od

MERLIN-TIMI 36 • Randomized, placebo controlled tiral. • Subjects: 6560 patients hospitalized with NSTEMI were randomized to ranolazine or placebo, in addition to standard therapy. • Initially ranolazine was given intravenous infusion followed by oral ranolazine. • Median duration of c. ECG monitoring was 6. 8 days. Circulation 2007; 116: 1647 -1652.

MERLIN-TIMI 36: SUMMARY • In more than 6300 patients admitted with NSTEMI, treatment with ranolzine resulted in significantly lower incidence of – ventricular tachycardia, – Supraventricular tachycardia, and – Significant ventricular pauses. Circulation 2007; 116: 1647 -1652.

Summary—Anti-Anginal and Anti-Ischemic Efficacy of Ranolazine • Dose and plasma concentration dependent • Consistent throughout a broad population of chronic angina patients • Not dependent on decreases in blood pressure or heart rate • At least as great as atenolol 100 mg qd (RAN 080) • In patients on atenolol or diltiazem at doses considered optimal by their physicians (RAN 072)

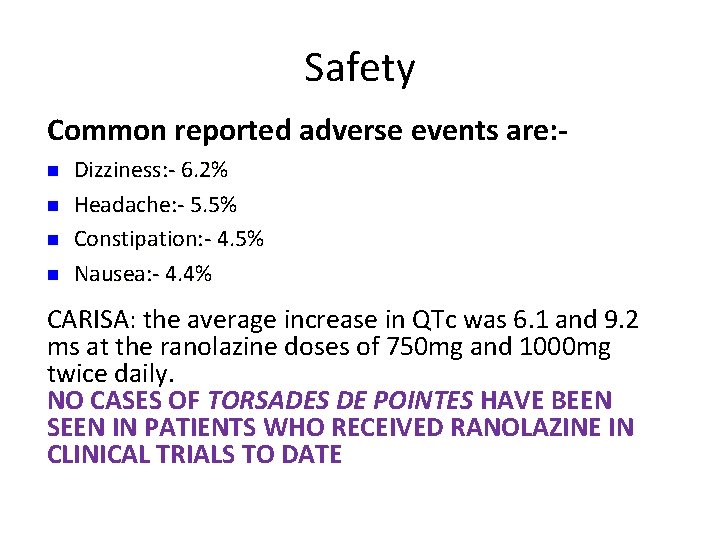
Safety Common reported adverse events are: n n Dizziness: - 6. 2% Headache: - 5. 5% Constipation: - 4. 5% Nausea: - 4. 4% CARISA: the average increase in QTc was 6. 1 and 9. 2 ms at the ranolazine doses of 750 mg and 1000 mg twice daily. NO CASES OF TORSADES DE POINTES HAVE BEEN SEEN IN PATIENTS WHO RECEIVED RANOLAZINE IN CLINICAL TRIALS TO DATE

Contraindications • • • Preexisting QT prolongation On drugs that prolong QT interval Hepatic impairment Patients taking drugs which inhibit CYP 3 A. In patients on potent and moderately potent CYP 3 A inhibitors such as ketoconazole, diltiazem, verapamil, macrolide antibiotics, HIV protease inhibitors, and grapefruit juice.

Indications & Dosage • Treatment of Chronic angina. • Patients who have not achieved an adequate response with other antianginal drug. • It should be used in combination with betablockers, amlodipine, or nitrates. • 500 mg bid initially, can be increased to 1000 mg bid. • Max. recommended daily dose is 1000 mg bid. • Helps in lowering Hb. A 1 c in patients with DM

Summary— Ranolazine Efficacy and Safety • Efficacy demonstrated in 5 double-blind, randomized, placebo-controlled trials • Safe and well tolerated • Adverse events are generally dose dependent and manageable by typical dose titration • No evidence for an adverse effect of ranolazine on survival

Caroza: A Novel Key For Angina