Randomized Controlled Trial Randomized Controlled Trial Aim testing










- Slides: 17

Randomized Controlled Trial

Randomized Controlled Trial Aim: testing medicines or medical procedures use randomized control �the most reliable form of scientific evidence �eliminates all forms of spurious causality Special condition: �randomized clinical trial (RCT) �clinical trial The basic idea: treatments are allocated to subjects at random different treatment groups are 'statistically equivalent'

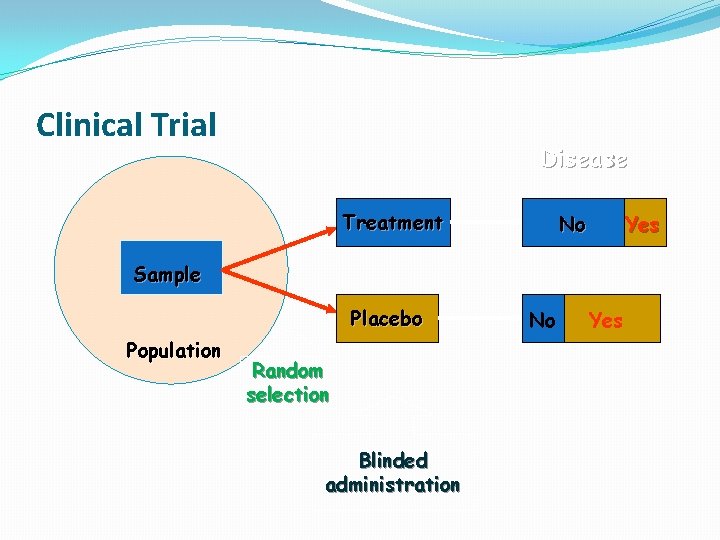
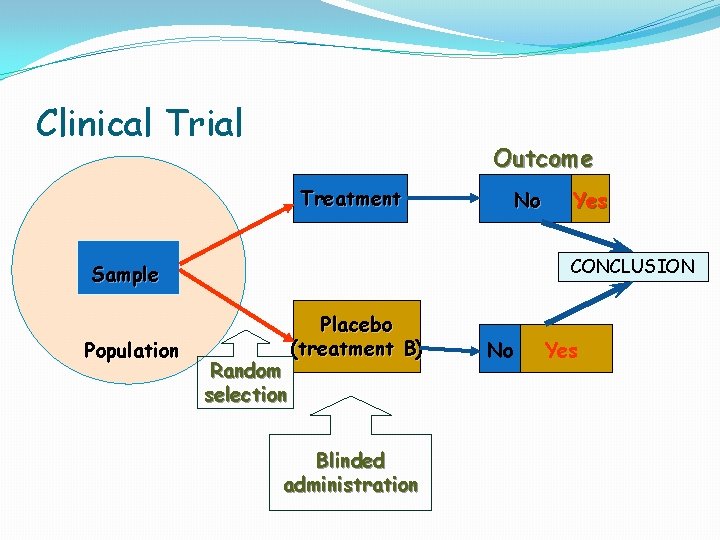
Clinical Trial Disease Treatment No Yes Sample Placebo Population Random selection Blinded administration No Yes

Randomized Controlled Trial STEPS: �Selection of participants �Random allocation to treatment or control arm �Blinding of treatment or control to patient and/or clinician

Randomized Controlled Trial SAMPLING: �Sample size calculation �Inclusion/exclusion criteria �Informed consent

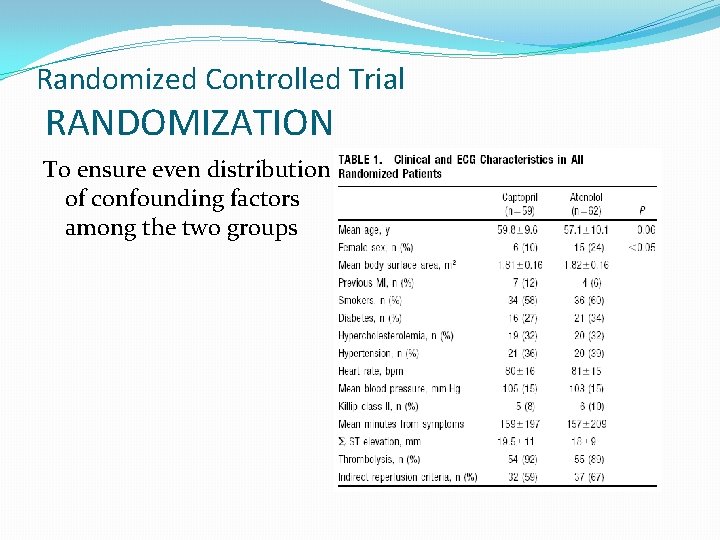
Randomized Controlled Trial RANDOMIZATION To ensure even distribution of confounding factors among the two groups

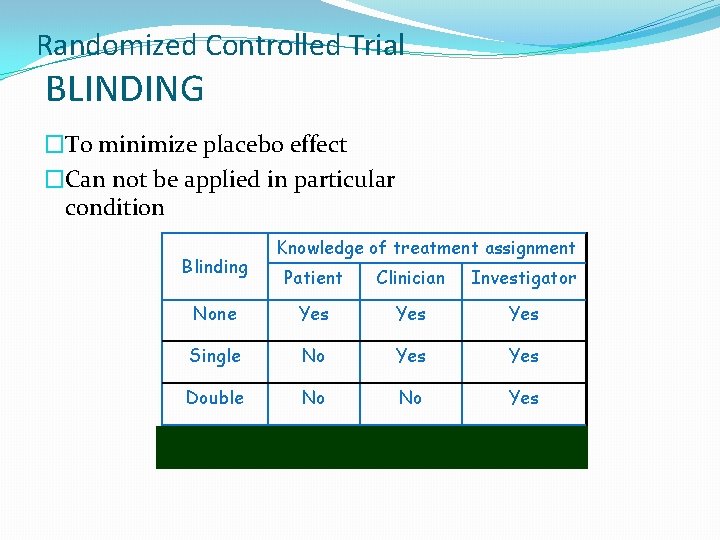
Randomized Controlled Trial BLINDING �To minimize placebo effect �Can not be applied in particular condition Blinding Knowledge of treatment assignment Patient Clinician Investigator None Yes Yes Single No Yes Double No No Yes Triple No No No


Randomized Controlled Trial ETHICAL ISSUE �Adopt new treatment without conducting experimental trial �Continue a trial if treatment harmful What if treatment obviously effective? �Conduct a trial with marginal benefit

Randomized Controlled Trial Advantages �Demonstrate causality �Randomization: eliminate the influence of confounding variables �Blinding : decreases bias Disadvantages �Expensive �Compliance �Selective population �Ethical issue

Randomized Controlled Trial PHASES Developed by FDA for approval of new treatment After experimental trial in vitro and in animal Testing a new treatment in small group of volunteer (usually healthy people) 1. • Determine: safe dose-range, side-effect Testing to a larger group (usually RCT) 2. • Determine effectiveness, tolerability, and safety

Randomized Controlled Trial PHASES (cont…) Test the treatment in a larger group of patients 3. Confirm effectiveness Monitor side effect Comparing with ‘old’ treatment Collect information for safe use of drug • • 4. Post marketing � � Effect on different population Side-effect

CONSIDER……. In preparing or using the results of RCT, Quality: � Reliability: replicable under ideal condition � Validity: the degree to which a study reaches a correct conclusion, depends on: � � Method Sample � Bias: systematic distortion of statistical result � Selection bias � Observer bias � Recall bias � Reporting bias � Publication bias

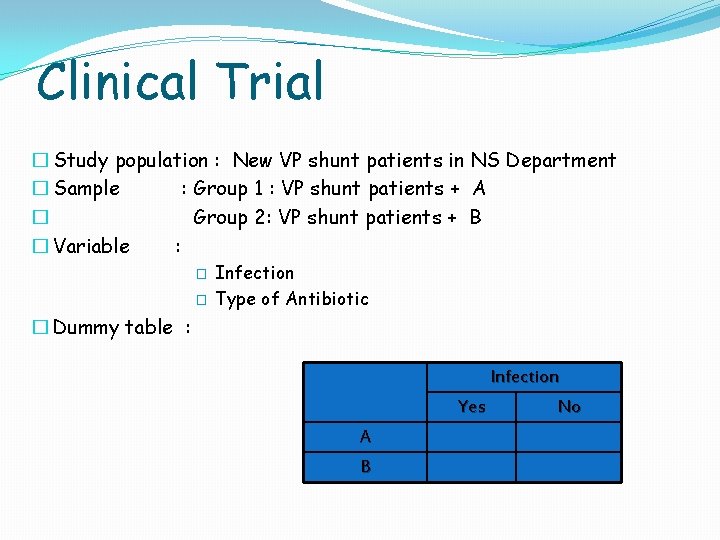
Clinical Trial � Study population : New VP shunt patients in NS Department � Sample : Group 1 : VP shunt patients + A � Group 2: VP shunt patients + B � Variable : � � Infection Type of Antibiotic � Dummy table : Infection Yes A B No

Clinical Trial Outcome Treatment No CONCLUSION Sample Population Yes Random selection Placebo (treatment B) Blinded administration No Yes

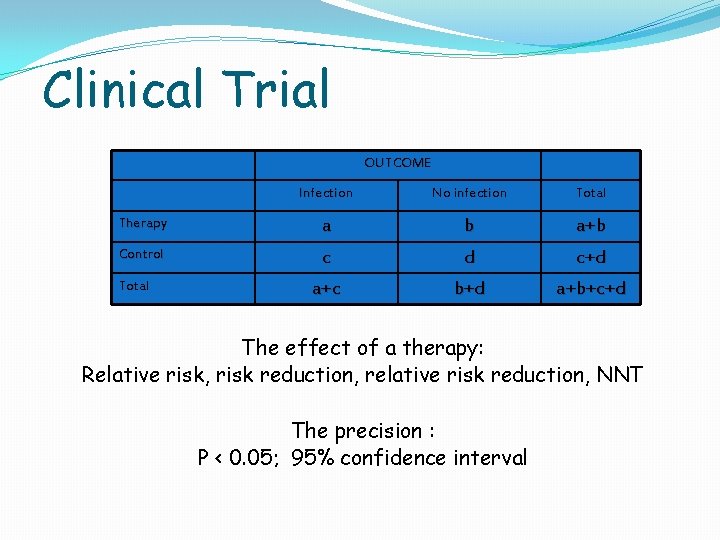
Clinical Trial OUTCOME Infection No infection Total Therapy a b a+b Control c d c+d a+c b+d a+b+c+d Total The effect of a therapy: Relative risk, risk reduction, relative risk reduction, NNT The precision : P < 0. 05; 95% confidence interval
