Randomized Comparison of Manual Compression and Use of





- Slides: 22

Randomized Comparison of Manual Compression and Use of Femo. Seal Vascular Closure Device for Closure after Femoral Artery Access Coronary Angiography CLOSE-UP The CLOSure d. Evices Used in everyday Practice study Niels Ramsing Holm, Birthe Sindberg, Mia Schou, Michael Maeng, Anne Kaltoft, Morten Bøttcher, Lars Romer Krusell, Leif Thuesen, Christian Juhl Terkelsen, Evald Høj Christiansen, Hans Erik Bøtker, Steen Dalby Kristensen and Jens Flensted Lassen For the CLOSE-UP study group

Potential conflicts of interest Speaker’s name: Niels R. Holm I have the following potential conflicts of interest to report: X Research contracts; St. Jude Medical �Consulting �Employment in industry �Stockholder of a healthcare company �Owner of a healthcare company X Other(s) Educational grant; St. Jude Medical niels. holm@ki. au. dk

Potential conflicts of interest The study was supported by unrestricted research grants from Vingmed Danmark A/S and St. Jude Medical niels. holm@ki. au. dk

CLOSE-UP: CLOSE-UP Background • Vascular Closure Devices (VCD) for femoral access CAG? • Debatable safety advantages • Superior efficacy • Cost-effectiveness? • Femo. Seal VCD safe in SCAAR registry* – no randomized trial *http: //www. ucr. uu. se/scaar/index. php/arsrapporter (2007)

CLOSE-UP: CLOSE-UP Aim Compare safety and efficacy of Femo. Seal and Manual Compression (MC) in a randomized trial

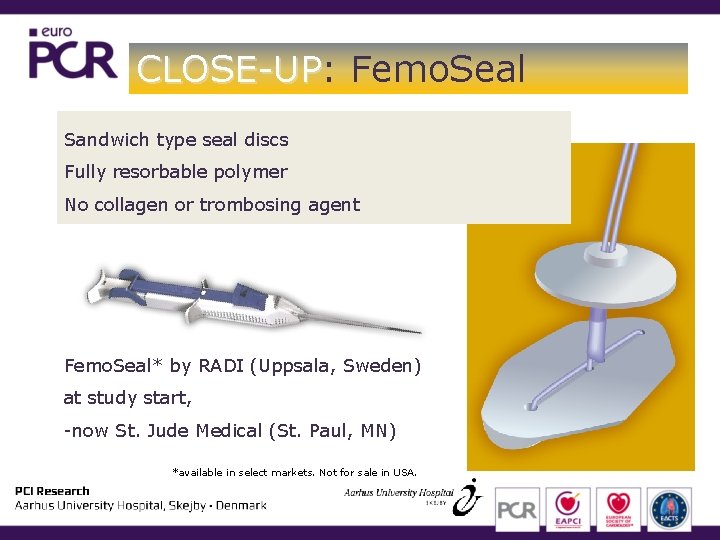
CLOSE-UP: CLOSE-UP Femo. Seal Sandwich type seal discs Fully resorbable polymer No collagen or trombosing agent Femo. Seal* by RADI (Uppsala, Sweden) at study start, -now St. Jude Medical (St. Paul, MN) *available in select markets. Not for sale in USA.

CLOSE-UP: CLOSE-UP Methods • Investigator initiated, driven and concluded • Randomized trial • Single high-volume center • CAG patients – No invasive diagnostics or therapy • In-hospital clinical evaluation • 14 days follow-up

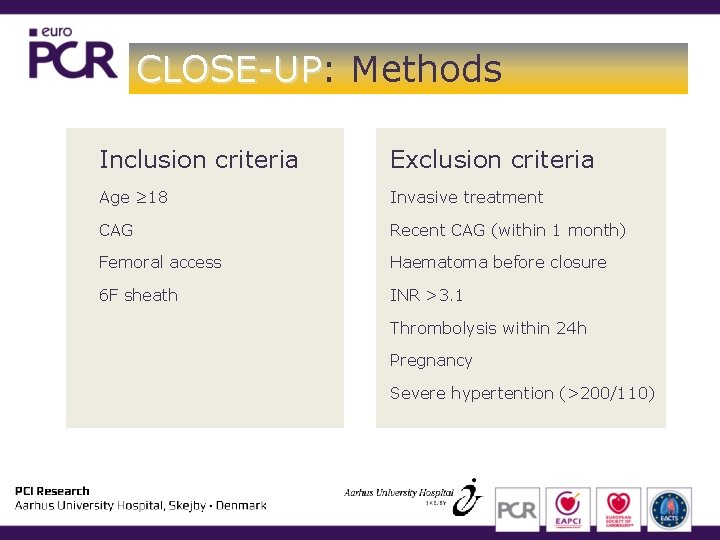
CLOSE-UP: CLOSE-UP Methods Inclusion criteria Exclusion criteria Age ≥ 18 Invasive treatment CAG Recent CAG (within 1 month) Femoral access Haematoma before closure 6 F sheath INR >3. 1 Thrombolysis within 24 h Pregnancy Severe hypertention (>200/110)

CLOSE-UP: CLOSE-UP Treatment Best practice CAG MC Immediate sheat removal Compression to haemostasis (at least 5 min) Sandbag discouraged in standard care No bandage or compression system used in standard care One hour bedrest recommended in both groups

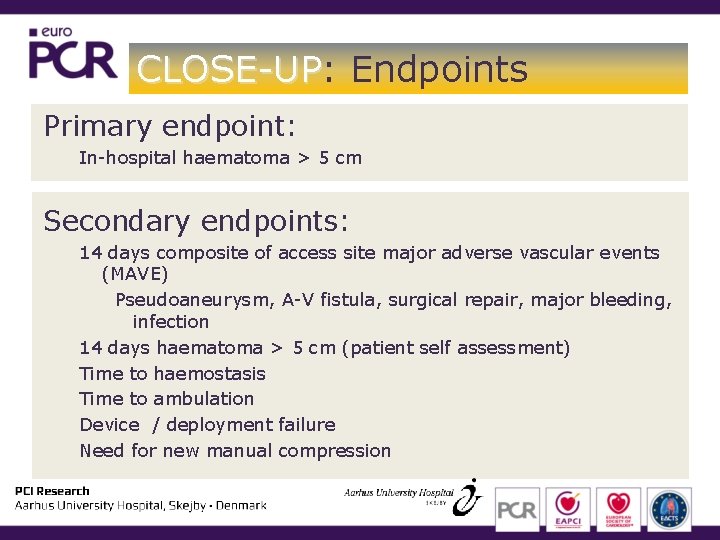
CLOSE-UP: CLOSE-UP Endpoints Primary endpoint: In-hospital haematoma > 5 cm Secondary endpoints: 14 days composite of access site major adverse vascular events (MAVE) Pseudoaneurysm, A-V fistula, surgical repair, major bleeding, infection 14 days haematoma > 5 cm (patient self assessment) Time to haemostasis Time to ambulation Device / deployment failure Need for new manual compression

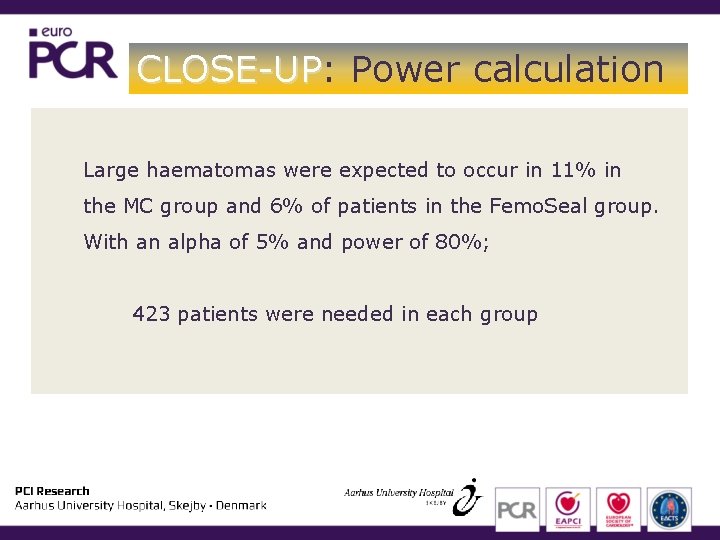
CLOSE-UP: CLOSE-UP Power calculation Large haematomas were expected to occur in 11% in the MC group and 6% of patients in the Femo. Seal group. With an alpha of 5% and power of 80%; 423 patients were needed in each group

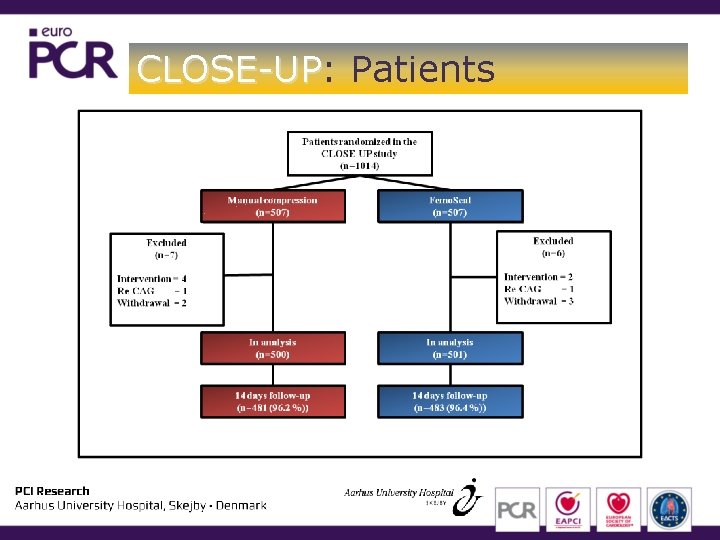
CLOSE-UP: CLOSE-UP Patients

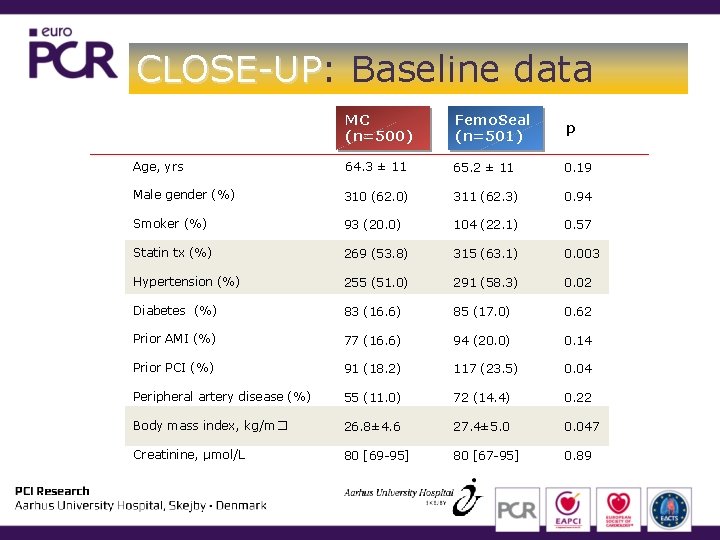
CLOSE-UP: CLOSE-UP Baseline data MC (n=500) Femo. Seal (n=501) p Age, yrs 64. 3 ± 11 65. 2 ± 11 0. 19 Male gender (%) 310 (62. 0) 311 (62. 3) 0. 94 Smoker (%) 93 (20. 0) 104 (22. 1) 0. 57 Statin tx (%) 269 (53. 8) 315 (63. 1) 0. 003 Hypertension (%) 255 (51. 0) 291 (58. 3) 0. 02 Diabetes (%) 83 (16. 6) 85 (17. 0) 0. 62 Prior AMI (%) 77 (16. 6) 94 (20. 0) 0. 14 Prior PCI (%) 91 (18. 2) 117 (23. 5) 0. 04 Peripheral artery disease (%) 55 (11. 0) 72 (14. 4) 0. 22 Body mass index, kg/m� 26. 8± 4. 6 27. 4± 5. 0 0. 047 Creatinine, μmol/L 80 [69 -95] 80 [67 -95] 0. 89

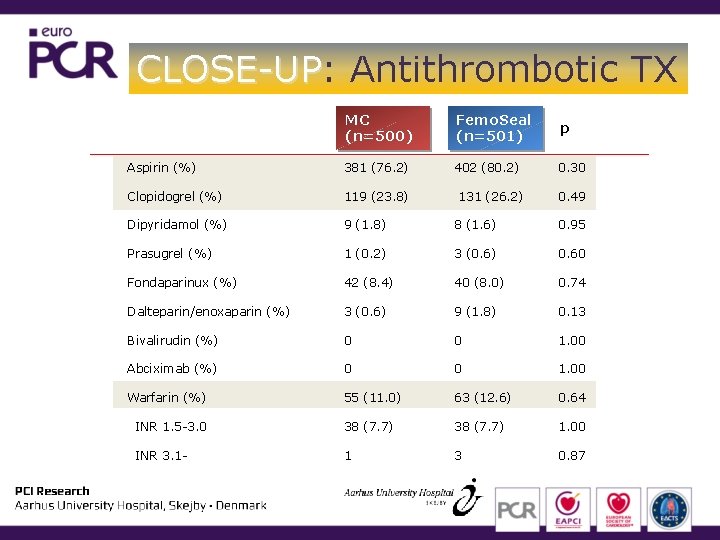
CLOSE-UP: CLOSE-UP Antithrombotic TX MC (n=500) Femo. Seal (n=501) p Aspirin (%) 381 (76. 2) 402 (80. 2) 0. 30 Clopidogrel (%) 119 (23. 8) Dipyridamol (%) 9 (1. 8) 8 (1. 6) 0. 95 Prasugrel (%) 1 (0. 2) 3 (0. 6) 0. 60 Fondaparinux (%) 42 (8. 4) 40 (8. 0) 0. 74 Dalteparin/enoxaparin (%) 3 (0. 6) 9 (1. 8) 0. 13 Bivalirudin (%) 0 0 1. 00 Abciximab (%) 0 0 1. 00 Warfarin (%) 55 (11. 0) 63 (12. 6) 0. 64 INR 1. 5 -3. 0 38 (7. 7) 1. 00 INR 3. 1 - 1 3 0. 87 131 (26. 2) 0. 49

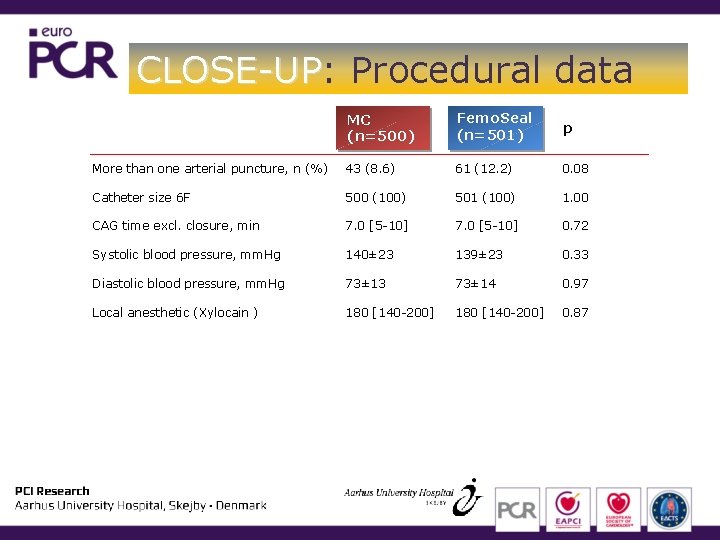
CLOSE-UP: CLOSE-UP Procedural data MC (n=500) Femo. Seal (n=501) p More than one arterial puncture, n (%) 43 (8. 6) 61 (12. 2) 0. 08 Catheter size 6 F 500 (100) 501 (100) 1. 00 CAG time excl. closure, min 7. 0 [5 -10] 0. 72 Systolic blood pressure, mm. Hg 140± 23 139± 23 0. 33 Diastolic blood pressure, mm. Hg 73± 13 73± 14 0. 97 Local anesthetic (Xylocain ) 180 [140 -200] 0. 87

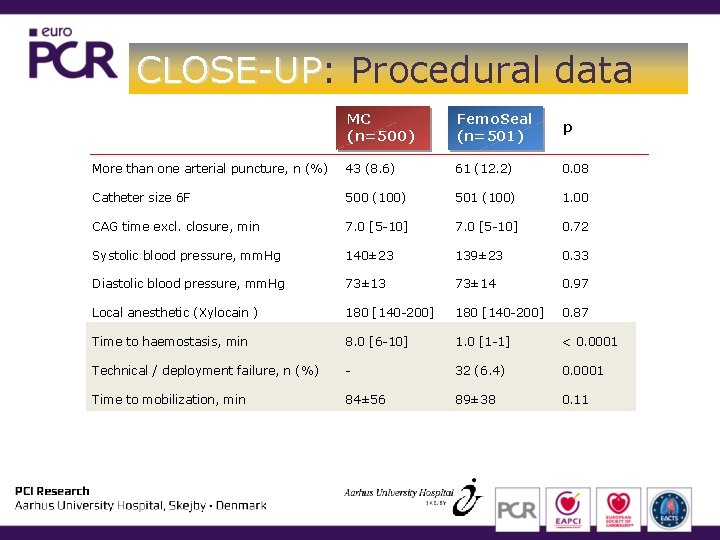
CLOSE-UP: CLOSE-UP Procedural data MC (n=500) Femo. Seal (n=501) p More than one arterial puncture, n (%) 43 (8. 6) 61 (12. 2) 0. 08 Catheter size 6 F 500 (100) 501 (100) 1. 00 CAG time excl. closure, min 7. 0 [5 -10] 0. 72 Systolic blood pressure, mm. Hg 140± 23 139± 23 0. 33 Diastolic blood pressure, mm. Hg 73± 13 73± 14 0. 97 Local anesthetic (Xylocain ) 180 [140 -200] 0. 87 Time to haemostasis, min 8. 0 [6 -10] 1. 0 [1 -1] < 0. 0001 Technical / deployment failure, n (%) - 32 (6. 4) 0. 0001 Time to mobilization, min 84± 56 89± 38 0. 11

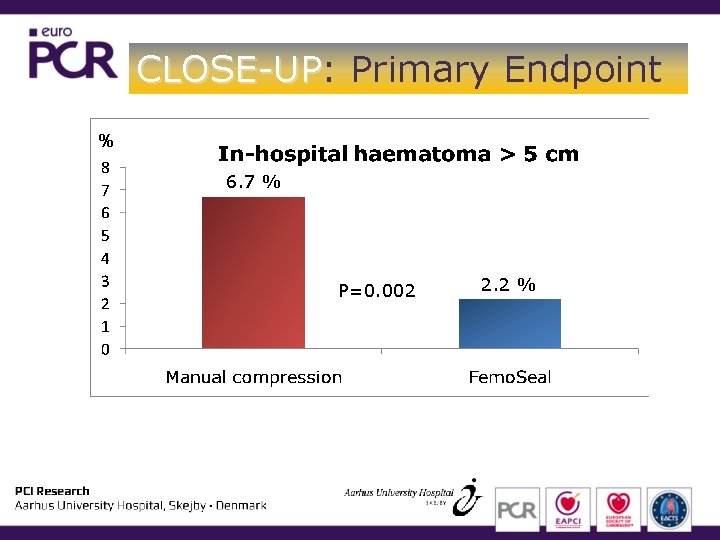
CLOSE-UP: CLOSE-UP Primary Endpoint

CLOSE-UP: CLOSE-UP Primary Endpoint % 6. 7 % P=0. 002 2. 2 %

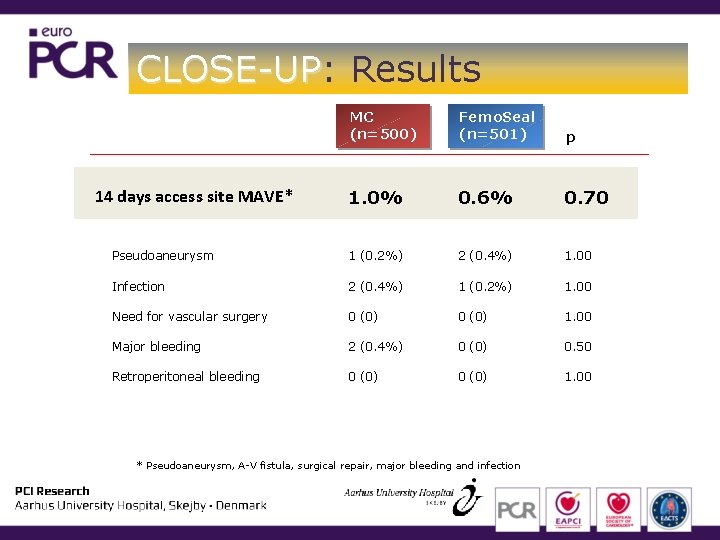
CLOSE-UP: CLOSE-UP Results MC (n=500) Femo. Seal (n=501) p 1. 0% 0. 6% 0. 70 Pseudoaneurysm 1 (0. 2%) 2 (0. 4%) 1. 00 Infection 2 (0. 4%) 1 (0. 2%) 1. 00 Need for vascular surgery 0 (0) 1. 00 Major bleeding 2 (0. 4%) 0 (0) 0. 50 Retroperitoneal bleeding 0 (0) 1. 00 14 days access site MAVE* * Pseudoaneurysm, A-V fistula, surgical repair, major bleeding and infection

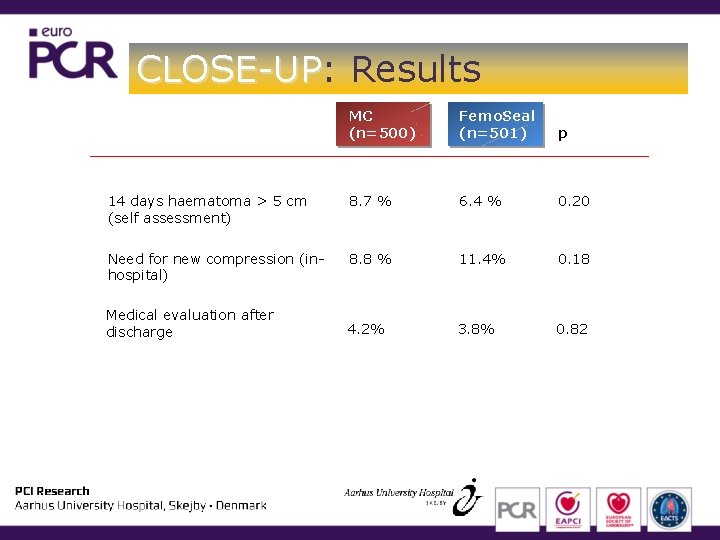
CLOSE-UP: CLOSE-UP Results MC (n=500) Femo. Seal (n=501) p 14 days haematoma > 5 cm (self assessment) 8. 7 % 6. 4 % 0. 20 Need for new compression (inhospital) 8. 8 % 11. 4% 0. 18 4. 2% 3. 8% 0. 82 Medical evaluation after discharge

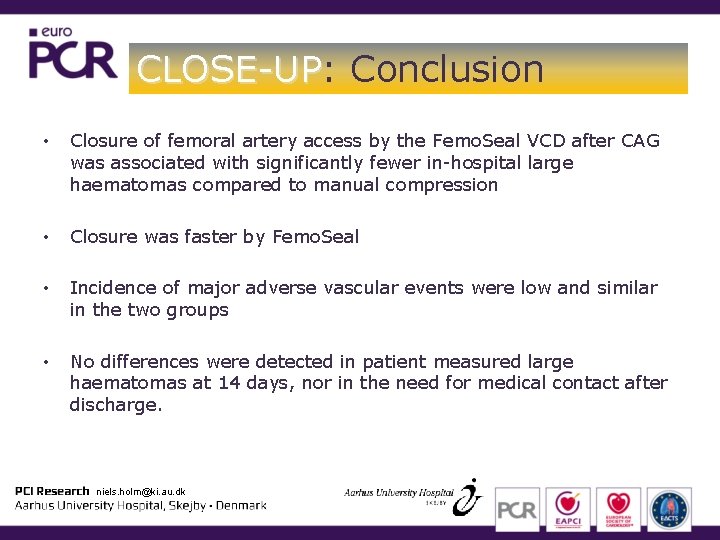
CLOSE-UP: CLOSE-UP Conclusion • Closure of femoral artery access by the Femo. Seal VCD after CAG was associated with significantly fewer in-hospital large haematomas compared to manual compression • Closure was faster by Femo. Seal • Incidence of major adverse vascular events were low and similar in the two groups • No differences were detected in patient measured large haematomas at 14 days, nor in the need for medical contact after discharge. niels. holm@ki. au. dk

CLOSE-UP: CLOSE-UP Conclusion • Closure of femoral artery access by the Femo. Seal VCD after CAG was associated with significantly fewer in-hospital large haematomas compared to manual compression • Closure was faster by Femo. Seal • Incidence of major adverse vascular events were low and similar in the two groups • No differences were detected in patient measured large haematomas at 14 days, nor in the need for medical contact after discharge. Acknowledgments to the staff at Dep. of Cardiology Aarhus University Hospital, Skejby, Denmark niels. holm@ki. au. dk