Radiopharmaceutical Production Target Foil Characteristics STOP Target Foils





- Slides: 12

Radiopharmaceutical Production Target Foil Characteristics STOP

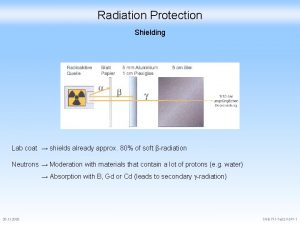
Target Foils • • In order to separate the target material from the vacuum of the cyclotron, a thin metal foil is often used on the front of a cyclotron target. This metal foil will attenuate the beam and therefore thin is better. On the other hand, the foil must be strong enough to withstand the pressure differential between the cyclotron vacuum and the target material. Contents • Thermal Conductivity • Tensile Strength • Chemical Reactivity • Energy Loss in the Foil • Activation of Foils STOP

Radiopharmaceutical Production Thermal Conductivity • Target Foils Contents Thermal Conductivity Tensile Strength Chemical Reactivity Energy Loss in the Foil Activation of Foils STOP • The thermal conductivity of the foil will determine the rate at which heat will be removed from the foil. If the foil is also cooled by either forced or free convection on the front surface (not in a vacuum), the heat deposited by the beam will be removed by a combination of these two processes. Foil materials such as aluminum are very good thermal conductors. The thickness of the foil will also determine the amount of heat which can be removed by this process as is evident from examining the equation for heat transfer by conduction. A list of some common foil materials and thermal conductivity for each is given in the Table on the next page.

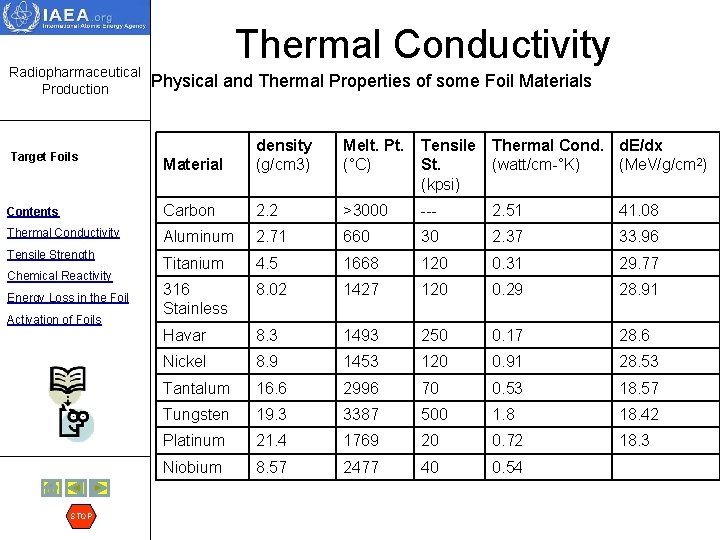
Radiopharmaceutical Production Thermal Conductivity Physical and Thermal Properties of some Foil Materials Material density (g/cm 3) Melt. Pt. (°C) Tensile Thermal Cond. d. E/dx St. (watt/cm-°K) (Me. V/g/cm 2) (kpsi) Contents Carbon 2. 2 >3000 --- 2. 51 41. 08 Thermal Conductivity Aluminum 2. 71 660 30 2. 37 33. 96 Titanium 4. 5 1668 120 0. 31 29. 77 316 Stainless 8. 02 1427 120 0. 29 28. 91 Havar 8. 3 1493 250 0. 17 28. 6 Nickel 8. 9 1453 120 0. 91 28. 53 Tantalum 16. 6 2996 70 0. 53 18. 57 Tungsten 19. 3 3387 500 1. 8 18. 42 Platinum 21. 4 1769 20 0. 72 18. 3 Niobium 8. 57 2477 40 0. 54 Target Foils Tensile Strength Chemical Reactivity Energy Loss in the Foil Activation of Foils STOP

Radiopharmaceutical Production Target Foils Contents Thermal Conductivity Tensile Strength Chemical Reactivity Energy Loss in the Foil Activation of Foils STOP Thermal Conductivity • As an illustration of the effects that convective cooling can have on the temperature of a foil, a simulation has been carried out and is presented in the Table. Increasing the film coefficient (h) decreases the temperature of the foil so that it can withstand higher beam currents. Havar was chosen as an example because thermal conductivity is low which means that convective cooling must be the primary means of heat removal. (See the section on heat transfer) The blanks in the table means the temperature was above the melting point of Havar at 1493°C. beam current (µA) Power density (watts/cm 2) Foil Temperature (°C) h=0. 01 h=0. 03 h=0. 06 20 15. 3 --- 1114 484 240 40 30. 6 --- 936 491 60 45. 8 --- 1331 735 80 61. 1 --- --- 973 100 76. 4 --- --- 1199

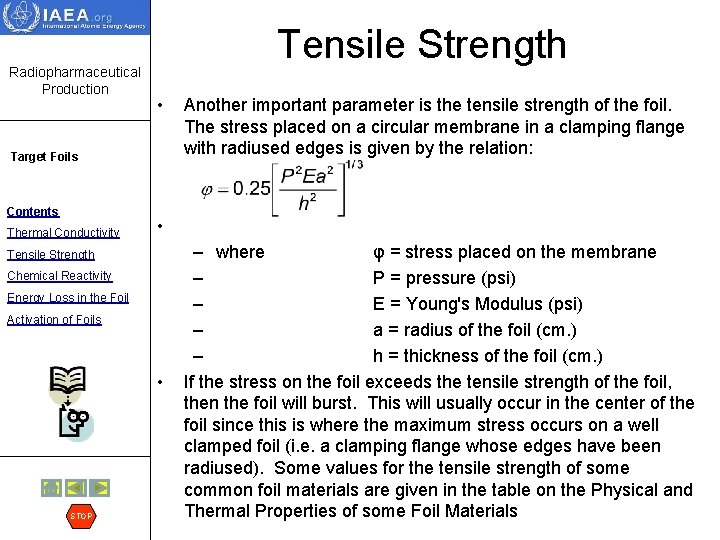
Radiopharmaceutical Production Tensile Strength • Target Foils Contents Thermal Conductivity • Tensile Strength Chemical Reactivity Energy Loss in the Foil Activation of Foils • STOP Another important parameter is the tensile strength of the foil. The stress placed on a circular membrane in a clamping flange with radiused edges is given by the relation: – where φ = stress placed on the membrane – P = pressure (psi) – E = Young's Modulus (psi) – a = radius of the foil (cm. ) – h = thickness of the foil (cm. ) If the stress on the foil exceeds the tensile strength of the foil, then the foil will burst. This will usually occur in the center of the foil since this is where the maximum stress occurs on a well clamped foil (i. e. a clamping flange whose edges have been radiused). Some values for the tensile strength of some common foil materials are given in the table on the Physical and Thermal Properties of some Foil Materials

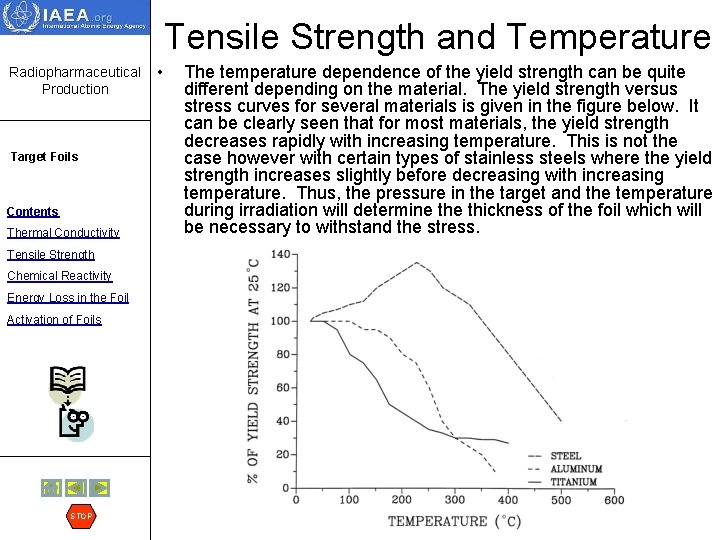
Tensile Strength and Temperature Radiopharmaceutical Production Target Foils Contents Thermal Conductivity Tensile Strength Chemical Reactivity Energy Loss in the Foil Activation of Foils STOP • The temperature dependence of the yield strength can be quite different depending on the material. The yield strength versus stress curves for several materials is given in the figure below. It can be clearly seen that for most materials, the yield strength decreases rapidly with increasing temperature. This is not the case however with certain types of stainless steels where the yield strength increases slightly before decreasing with increasing temperature. Thus, the pressure in the target and the temperature during irradiation will determine thickness of the foil which will be necessary to withstand the stress.

Radiopharmaceutical Production Chemical Reactivity • Target Foils • Contents Thermal Conductivity Tensile Strength Chemical Reactivity • Energy Loss in the Foil Activation of Foils STOP • The next important characteristic of the foil is the chemical reactivity. This depends on the target material. In nitrogen targets, the foil is often aluminum since this material is chemically inert to the nitrogen gas and to the carbon-11 products produced. Aluminum cannot be used in a target for the production of fluorine-18 from oxygen-18 water since the fluorine interacts with the aluminum and it is very difficult to remove the fluorine 18 from the target. An aluminum target can be used for gaseous fluorine-18 production since the surface can be made non-reactive by exposure to fluorine gas at low concentrations. It is necessary to consider the chemical combination of the foil material with the target material not only at room temperature but also at elevated temperatures since this is often the situation inside the target. Each target must be considered on a case by case basis and there are no rules other than those of chemistry.

Radiopharmaceutical Production Target Foils Energy Loss in the Foil • • Contents Thermal Conductivity Tensile Strength Chemical Reactivity Energy Loss in the Foil Activation of Foils STOP • The energy loss in the foil is another consideration, since this will have an impact on the beam energy incident on the target material and also on the heat which is deposited in the foil. The energy degradation relates to the stopping power of the material as was calculated in the section on physics. The ideal is to have a foil as thin as possible to withstand the pressure in the target so that the minimum amount of energy is deposited in the foil. An exception to this rule comes up when it is necessary to reduce the beam energy in order to have the energy incident on the target material at an optimum energy with respect to the cross-section of the desired nuclear reaction.

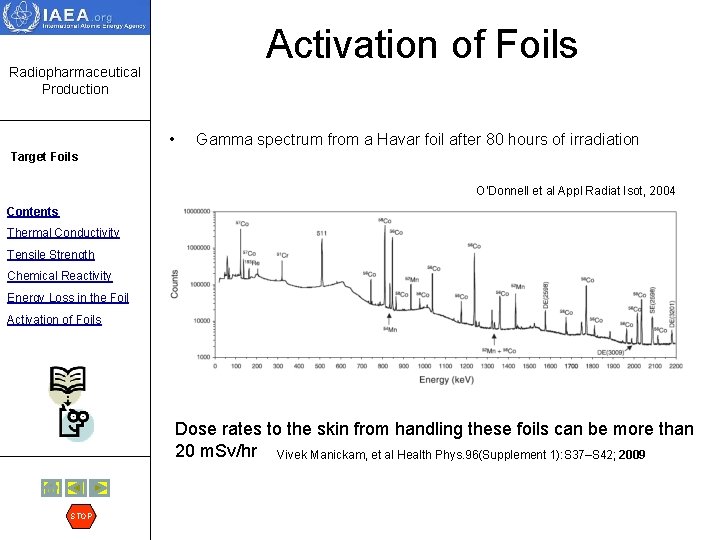
Radiopharmaceutical Production Activation of Foils • Target Foils Contents Thermal Conductivity • Tensile Strength Chemical Reactivity Energy Loss in the Foil Activation of Foils • STOP Another consideration is the radioactivation of the target foils, since this will often determine how radioactive the target will be. All target foils need to be replaced at fairly frequent intervals and this can result in a radiation dose to the person working on the target. Aluminum is often the material of choice in this regard because there are very few long lived activities formed in the foil. Nickel alloys and steels, which must be used for chemical inertness in certain situations, are perhaps the worst commonly used materials with respect to activation since these metals often have several long-lived activities associated with them. One of the most common foils used for cyclotron targets is Havar and it has many activation products. A gamma spectrum is shown on the next slide.

Activation of Foils Radiopharmaceutical Production • Gamma spectrum from a Havar foil after 80 hours of irradiation Target Foils O’Donnell et al Appl Radiat Isot, 2004 Contents Thermal Conductivity Tensile Strength Chemical Reactivity Energy Loss in the Foil Activation of Foils Dose rates to the skin from handling these foils can be more than 20 m. Sv/hr Vivek Manickam, et al Health Phys. 96(Supplement 1): S 37–S 42; 2009 STOP

Return to Main Menu
 Dmsa test
Dmsa test What is a radiopharmaceutical
What is a radiopharmaceutical Radiopharmaceutical
Radiopharmaceutical Contoh pre production
Contoh pre production Aperture stop
Aperture stop One stop teacher stop
One stop teacher stop Production stop
Production stop The 3 apparitions in scene 1 leave macbeth feeling
The 3 apparitions in scene 1 leave macbeth feeling Unlike hamlet, laertes is __________.
Unlike hamlet, laertes is __________. What are foils in literature
What are foils in literature King lear characters
King lear characters Foils in literature are
Foils in literature are Foil characters in romeo and juliet
Foil characters in romeo and juliet